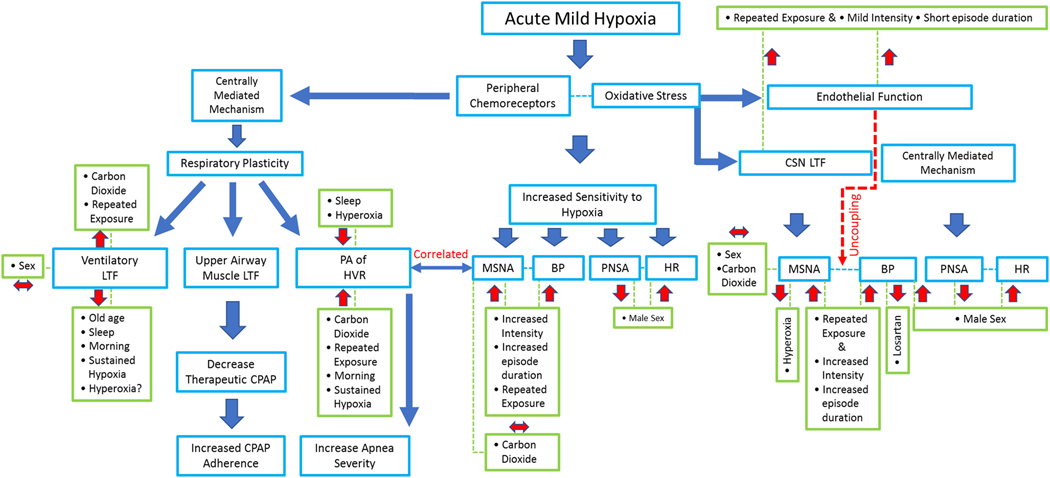
Abstract
This review explores forms of respiratory and autonomic plasticity, and associated outcome measures, that are initiated by exposure to intermittent hypoxia. The review focuses primarily on studies that have been completed in humans and primarily explores the impact of mild intermittent hypoxia on outcome measures. Studies that have explored two forms of respiratory plasticity, progressive augmentation of the hypoxic ventilatory response and long-term facilitation of ventilation and upper airway muscle activity, are initially reviewed. The role these forms of plasticity might have in sleep disordered breathing are also explored. Thereafter, the role of intermittent hypoxia in the initiation of autonomic plasticity is reviewed and the role this form of plasticity has in cardiovascular and hemodynamic responses during and following intermittent hypoxia is addressed. The role of these responses in individuals with sleep disordered breathing and spinal cord injury are subsequently addressed. Ultimately an integrated picture of the respiratory, autonomic and cardiovascular responses to intermittent hypoxia is presented. The goal of the integrated picture is to address the types of responses that one might expect in humans exposed to one-time and repeated daily exposure to mild intermittent hypoxia. This form of intermittent hypoxia is highlighted because of its potential therapeutic impact in promoting functional improvement and recovery in several physiological systems.
Keywords: Progressive augmentation, Long-term facilitation, Blood pressure, Peripheral chemoreflex
1. Introduction
This review is focused on respiratory, autonomic and cardiovascular responses that have been documented in humans following treatment with intermittent hypoxia. This stimulus has been shown to initiate beneficial outcomes when a mild/moderate dose of hypoxia is administered acutely (e.g. on a single visit to the laboratory) or repeatedly (e.g. multiple visits to the laboratory on consecutive days) (Mateika et al., 2015; Mateika and Komnenov, 2017). Defining mild/moderate intermittent hypoxia is difficult because there are several variables to be considered when developing a protocol for humans (Mateika and Sandhu, 2011; Navarrete-Opazo and Mitchell, 2014). These variables include, but are not limited to, the intensity of hypoxia, the number and duration of hypoxic episodes and the profile of the oxygen saturation/desaturation wave form (e.g. square wave vs. sinusoidal). To date, detailed dose response studies in humans, which could provide guidance in selecting the appropriate variables, have not been completed. Defining the intermittent nature of a protocol is more difficult since the time frame of exposure varies greatly in the literature. The time frame may range from a single bout of hypoxia each day versus exposure to repetitive episodes of hypoxia each day for several days, including protocols that increase the number and duration of cycles and the intensity of hypoxia over several weeks (Mateika and Sandhu, 2011). Moreover, the duration of these hypoxic bouts may range from two minutes to several hours. For example, therapeutic and therapeutic-like intermittent normobaric hypoxia protocols may be defined by thirty minutes of hypoxia each day for ten days, or twelve 2-min cycles of hypoxia interspersed with 2 min of normoxia administered each day for 15 days (Mateika and Sandhu, 2011). Typically, the protocols used to initiate two forms of respiratory plasticity in humans, which are outlined below, are comprised of a few episodes (e.g. 12 episodes) of short duration (e.g. 2 min) (Fig. 1). If repeated daily exposure is implemented, the exposure typically has not extended beyond 3 weeks. To add to the variable nature of the protocols used to initiate respiratory plasticity, those linked to autonomic and cardiovascular outcomes are even more variable. Given this conundrum, the information included in this review is based principally on the reporting of beneficial outcome measures rather than specific dose requirements. Consequently, details of specific protocols are typically not presented in the manuscript. Instead, as a service to our readers we have summarized the characteristics of each referenced protocol and the accompanying outcome measures in a supplemental file. Nonetheless, for those that require a distinct definition of mild/moderate hypoxia, we consider an oxygen saturation level at or above 85% fulfills the definition provided that the duration and number of hypoxic episodes are few. Although some respiratory, autonomic and cardiovascular outcomes linked to mild intermittent hypoxia are beneficial, detrimental outcomes are also outlined.
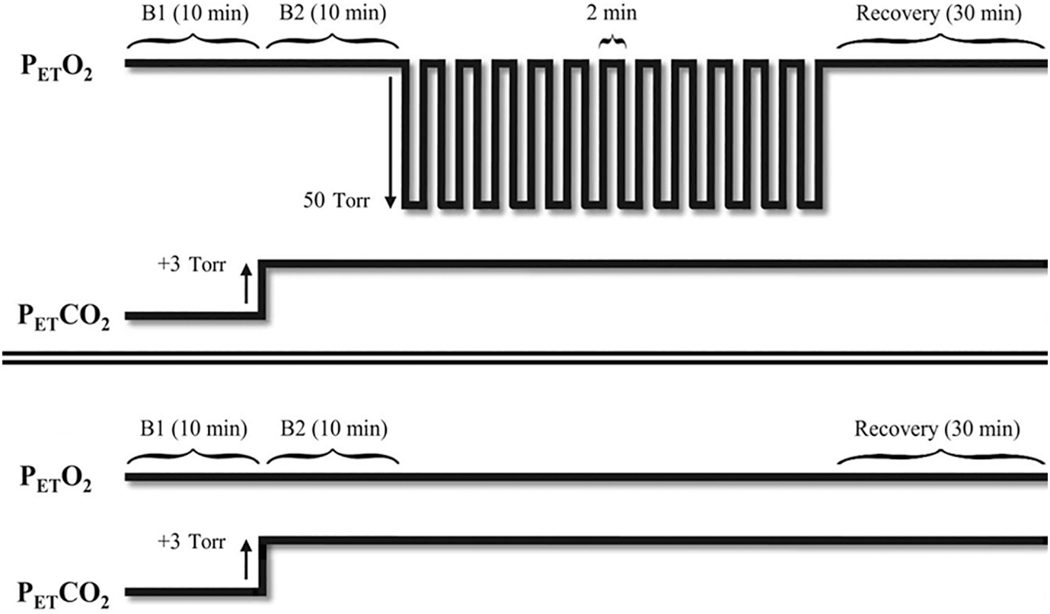
Fig. 1.
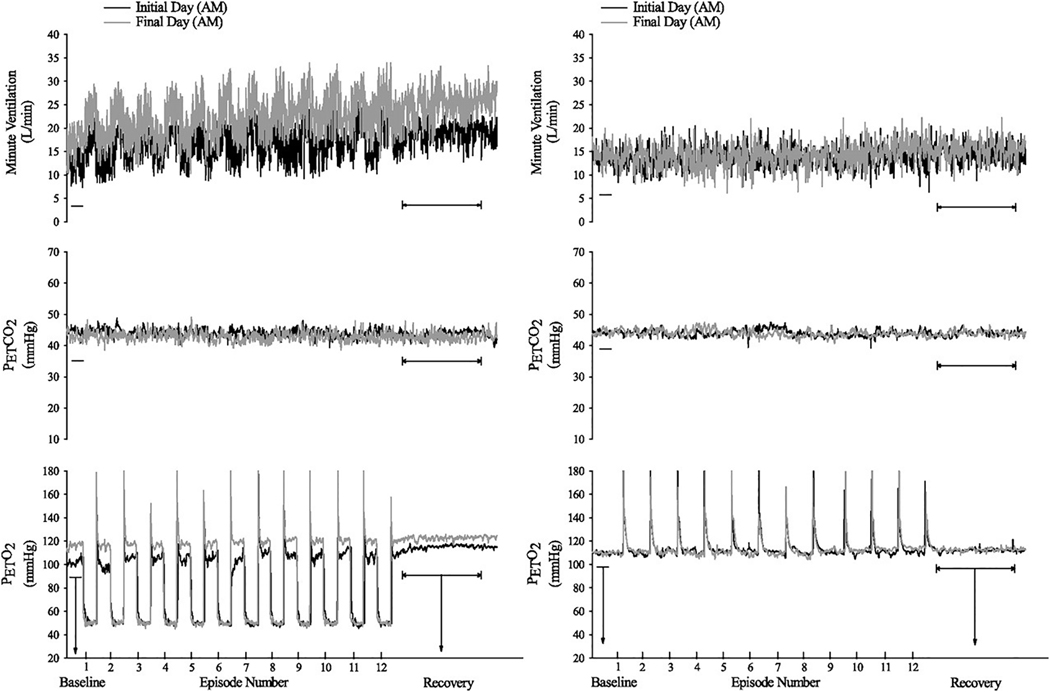
Example of an intermittent hypoxia (Top) and sham (Bottom) protocol. In this example 12 episodes of hypoxia that were two minutes in duration were separated by 2-min recovery periods with the exception of the final 30-min recovery period. PETCO2, partial pressure of end-tidal carbon dioxide; PETO2, partial pressure of end-tidal oxygen; B1, initial baseline period measured under normoxic conditions; B2, second baseline period measured under hypercapnic conditions. Reprinted from “The impact of arousal state, sex, and sleep apnea on the magnitude of progressive augmentation and ventilatory long- term facilitation” by Z. Syed et al. J. Appl. Physiol. 114: 52–65, 2013.
The review is directed toward experimentally induced intermittent hypoxia, however beneficial outcomes coupled to naturally occurring intermittent hypoxia that is characteristic of obstructive sleep apnea will also be mentioned. To establish context, findings obtained from animal models are mentioned, but we have purposely avoided an extensive review of the literature in this area to maintain focus on findings in human participants. In reviewing the respiratory, autonomic and cardiovascular outcomes, we will address factors that might influence the magnitude of the response including (i) the timing of administration (wake vs. sleep, morning vs. afternoon vs. evening) (ii) dose characteristics [e.g. single dose (i.e. one day) vs. repeated dose (daily repeated intermittent hypoxia), (iii) the role of carbon dioxide (if any) and (iv) anthropometric factors (e.g. sex, and age). In some cases, these factors will not be addressed or will not be addressed under a specific heading because published information is limited or not available. Furthermore, we excluded studies that combine hypoxia with other perturbations (i.e. hypoxia administered during exercise). Thus, the focus of the review is specific to normobaric hypoxia and removes potential confounders including exercise. For reviews that focus on hypobaric hypoxia, hypoxia and exercise, and, hypoxia and other perturbations, we direct the reviewers elsewhere (Hobbins et al., 2017; Mallet et al., 2018; Scott et al., 2014; Serebrovskaya et al., 2008; Viscor et al., 2018).
2. Respiratory plasticity
Plasticity is a fundamental characteristic of a physiological system and may be defined as the adaptive response of a system to a stimulus, or variation in environmental input, to establish homeostasis and/or stability (Dennis et al., 2013; Mitchell and Johnson, 2003). However, there can be maladaptive responses to the stimuli that result in impairment in the function and/or recovery of a physiological system (Velotta and Cheviron, 2018). Respiratory plasticity or respiratory motor neuronal plasticity is defined as the persistent morphological and/or functional modification of neural pathways and synapses of the nervous system involved in the control of breathing in response to chemical stimuli, exercise, ascent to altitude, injury or stress (Mitchell and Johnson, 2003). These adaptive responses to short-term or long-term perturbations in arousal state, metabolic demand or environment, for example, are crucial to maintain homeostatic arterial blood gases and ventilation. Respiratory plasticity can be expressed at the chemoreceptor (Prabhakar, 2011), brainstem (Morris et al., 2003), spinal (Dale-Nagle et al., 2010) and neuromuscular (Rowley et al., 2005) level of the control of breathing.
There are two forms of hypoxia-induced respiratory plasticity: progressive augmentation of the hypoxic ventilatory response and long- term facilitation (Mateika and Narwani, 2009; Mateika et al., 2018). Progressive augmentation is characterized by a progressive increase in the ventilatory response to hypoxia that occurs from the initial to the final episode of an intermittent hypoxia protocol (Fig. 2). Long-term facilitation is characterized by a gradual increase in respiratory motor output relative to baseline during successive periods of normoxia that separate hypoxic episodes, and the sustained elevation of the respiratory motor output may last for up to 90 min following removal of the hypoxic stimulus (Fig. 2) (Mateika and Narwani, 2009; Mateika et al., 2018; Mateika and Sandhu, 2011). In humans, acute intermittent hypoxia initiates a sustained increase in minute ventilation which is termed ventilatory long-term facilitation and is described below. The occurrence of progressive augmentation and long-term facilitation principally depends upon the paradigm of the hypoxic stimulus that includes the duration (acute vs. chronic), intensity (mild vs. severe) and pattern of hypoxia (intermittent vs. sustained). The following sections describe each form of respiratory plasticity in detail.
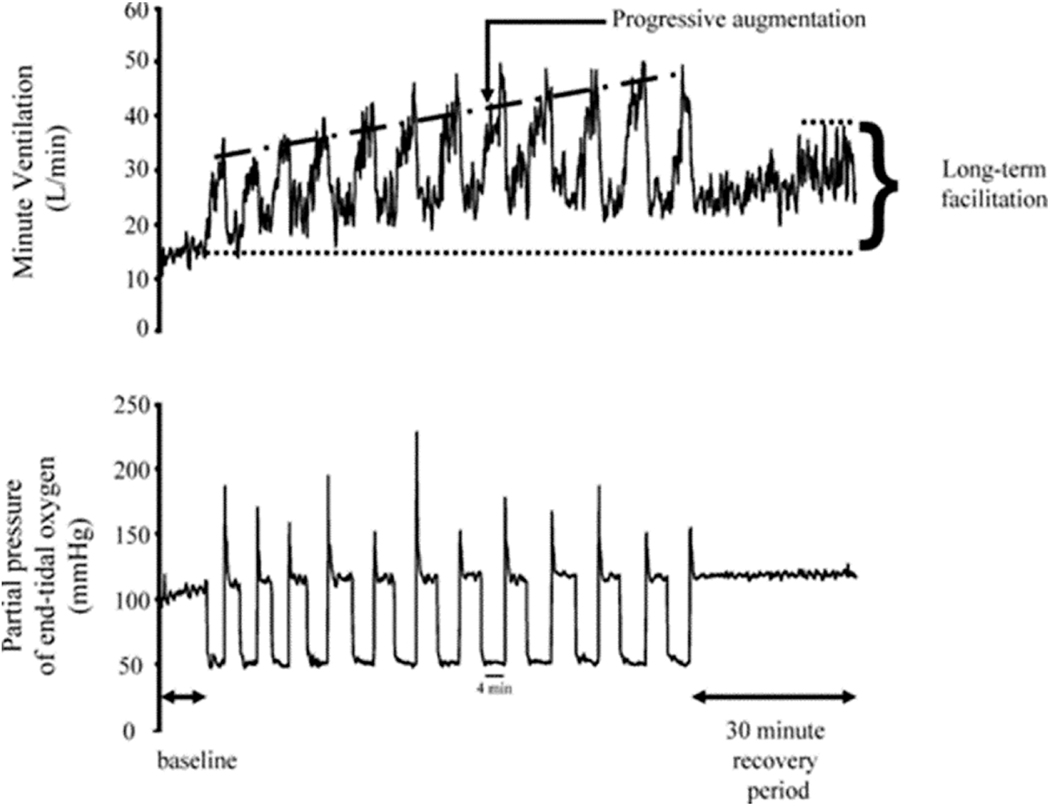
Fig. 2.
A recording of breath-by-breath minute ventilation values recorded from a human participant before, during and following exposure to 12 episodes of hypoxia Each hypoxic episode and subsequent recovery period was 4 min in duration with the exception of the last recovery period, which was 30 min in duration. Note that during exposure to intermittent hypoxia the ventilatory response to hypoxia gradually increased from the initial hypoxic episode to the final hypoxic episode. This phenomenon is referred to as progressive augmentation. Also note that during exposure to intermittent hypoxia minute ventilation gradually increased during the normoxic recovery periods so that it was substantially higher during the final recovery period compared with baseline. This phenomenon is referred to as ventilatory long-term facilitation. Reprinted with permission from “Intermittent hypoxia and respiratory plasticity in humans and other animals: does exposure to intermittent hypoxia promote or mitigate sleep apnoea,” by J.H. Mateika and G. Narwani. Exp. Physiol. 94: 279–296, 2009.
3. Progressive augmentation of the hypoxic ventilatory response
3.1. Manifestation of progressive augmentation
Progressive augmentation was originally observed in inspiratory intercostal nerve activity of cats (Fregosi and Mitchell, 1994) and has also been observed in the minute ventilation responses of awake goats and ducks (Mitchell et al., 2001b; Turner and Mitchell, 1997) and anesthetized rats (Nichols et al., 2012). Attempts to demonstrate progressive augmentation during the administration of intermittent hypoxia, in both healthy humans (Mateika et al., 2004; Morelli et al., 2004) and humans with sleep apnea (Khodadadeh et al., 2006), was initially unsuccessful leading to the possible conclusion that progressive augmentation is not a firmly established form of plasticity. On the other hand, the absence of progressive augmentation could be because carbon dioxide levels were not maintained at or above baseline levels (also see Ventilatory long-term facilitation for additional details regarding the impact of carbon dioxide on long term facilitation). Typically, hypocapnia is induced in response to hyperventilation initiated by hypoxia, which in turns serves to disfacilitate the response. In support of this speculation, a progressive increase in minute ventilation during exposure to intermittent hypoxia was evident when carbon dioxide levels were maintained above baseline in both healthy individuals (Harris et al., 2006; Syed et al., 2013; Wadhwa et al., 2008) and individuals with obstructive sleep apnea (Gerst III et al., 2011; Lee et al., 2009). Similarly, numerous studies have shown that the response to hypoxia soon after exposure to intermittent hypoxia was enhanced, compared to a sham protocol, when carbon dioxide levels were at or above baseline in healthy individuals (Mateika et al., 2004;Morelli et al., 2004) and individuals with obstructive sleep apnea (Gerst III et al., 2011; Khodadadeh et al., 2006).
There are other circumstances in which progressive augmentation during exposure to mild intermittent hypoxia may not be evident, even if carbon dioxide levels are maintained. Progressive augmentation may be difficult to observe at extreme stimulus intensities. In cats, progressive augmentation was less obvious in phrenic nerve activity compared to intercostal nerve activity, because the phrenic response to the initial hypoxic episode was greater than 90% of maximal activity (Fregosi and Mitchell, 1994). In other words, if a maximal response is initiated early on in an intermittent hypoxia protocol it is unlikely that progressive enhancement will be evident. On the other hand, work completed in rats suggests that if the intensity of the hypoxic stimulus is too mild, progressive augmentation will not be initiated. Nichols and colleagues reported in anesthetized rats that a partial pressure of oxygen equivalent to 25–30 mmHg led to the initiation of progressive augmentation (Nichols et al., 2012), while a more moderate stimulus (45–55 mmHg) under the same experimental conditions did not initiate the phenomenon (Nichols et al., 2012).
Even if a progressive increase is evident during administration of intermittent hypoxia it may be difficult to quantify. The hypoxic ventilatory response is determined by subtracting minute ventilation during the hypoxic episode from the minute ventilation measured during baseline (i.e. the recovery period from the previous hypoxic episode). The difference is divided by the change in oxygen saturation measured during baseline periods and the accompanying hypoxic episode. The issue in quantifying progressive augmentation is that measures of minute ventilation throughout the recovery periods of an intermittent hypoxia protocol also typically increase. Thus, it is difficult to ascertain in some cases if the progressive increase is the result of an enhanced response to hypoxia or the development of long-term facilitation. In these cases, measures of the hypoxia ventilatory response following exposure to an intermittent hypoxia protocol using a variety of different methods (e.g. modified rebreathing method, sustained isocapnic hypoxia) might help to differentiate the underlying phenomenon. Despite these concerns, there is sufficient evidence indicating that progressive augmentation of the hypoxic ventilatory response occurs in response to exposure to intermittent hypoxia in humans. From a translational perspective, enhancement of the ventilatory response to hypoxia could lead to the perpetuation of apneic events (see Progressive Augmentation, Long-Term Facilitation and Apnea Severity) (Dempsey et al., 2010; Mateika and Narwani, 2009; Mateika and Syed, 2013).
3.2. Mechanistic underpinnings of progressive augmentation
The site of origin of progressive augmentation might be the carotid bodies, since these receptors are the primary detectors of hypoxia (Duffin, 2007) and the ventilatory response to hypoxia both during or after repeated daily exposure to intermittent hypoxia is enhanced (Foster et al., 2009; Foster et al., 2005; Gerst III et al., 2011; Gilmartin et al., 2008; Koehle et al., 2007). Moreover, progressive augmentation of carotid sensory nerve activity has been recorded in rats (Peng et al., 2003; Roy et al., 2018).
Independent of the site of origin, the accumulation of reactive oxygen species might be the mechanistic link between intermittent hypoxia and the enhanced progressive augmentation observed in sleep apnea participants. This postulation is supported by findings which showed that chronic exposure to intermittent hypoxia led to an enhanced in vivo and ex vivo carotid body sensory response to hypoxia (Peng and Prabhakar, 2004). The augmented hypoxic carotid sensory response was abolished in a group of rats pre-treated with a potent scavenger of superoxide anions (Peng and Prabhakar, 2003, 2004). More importantly for the present review, measures of oxidative stress are correlated to loop gain (Panza et al., 2019) in humans. Loop gain is in part impacted by the sensitivity of the response to hypoxia (Puri et al., 2020). Likewise, increases in oxidative stress induced by repeated daily exposure to intermittent hypoxia were positively correlated to increases in the ventilatory response to hypoxia that occurred following 4 days of exposure to intermittent hypoxia (Pialoux et al., 2009). This finding was further supported by results which showed that administration of an antioxidant cocktail reduced progressive augmentation of the hypoxic ventilatory response both during and following an acute exposure to intermittent hypoxia in humans with obstructive sleep apnea (Lee et al., 2009).
In contrast, administration of a serotonin receptor antagonist to anesthetized cats (Fregosi and Mitchell, 1994) and rats (Nichols et al., 2012), in addition to administration of an adenosine receptor antagonist in rats (Nichols et al., 2012), did not impact the manifestation of this phenomenon, which is dissimilar to its impact on long-term facilitation (Fregosi and Mitchell, 1994; Nichols et al., 2012). However, it should be noted that a single study completed in rats indicated that the response of the recurrent laryngeal and phrenic nerve to an acute bout of hypoxia was enhanced 60 min following exposure to intermittent hypoxia (Bautista et al., 2012). This enhancement is indicative of progressive augmentation. The response was eliminated following the application of a serotonin receptor antagonist (Bautista et al., 2012), contrary to the previously cited investigations.
4. Ventilatory long-term facilitation
4.1. Manifestation of ventilatory long-term facilitation
Ventilatory long-term facilitation is defined by a persistent increase in minute ventilation and/or its components (tidal volume and breathing frequency) following exposure to acute intermittent hypoxia (Mateika and Narwani, 2009). Evidence from animal models indicate that exposure to intermittent hypoxia evokes modifications in the cell bodies of medullary respiratory and/or phrenic motor neurons. These modifications result in an increase in neural activity, ultimately leading to a sustained increase in ventilation after cessation of the hypoxic stimulus (Mitchell and Johnson, 2003).
Similar to progressive augmentation, results from early studies indicated that ventilatory long-term facilitation was not initiated in healthy humans (Jordan et al., 2002; McEvoy et al., 1997; Morelli et al., 2004) and in individuals with obstructive sleep apnea (Khodadadeh et al., 2006) following exposure to intermittent hypoxia during wakefulness. However, participants in these studies were exposed to poikilocapnic intermittent hypoxia that constrained ventilation via disfacilitation of chemoreceptor feedback through the induction of hypocapnia. Subsequent studies have shown that the manifestation of ventilatory long-term facilitation in humans during wakefulness requires the maintenance of carbon dioxide above baseline. Carbon dioxide levels were initially maintained 5 mmHg above baseline (Harris et al., 2006). However, long-term facilitation has also been evident when carbon dioxide is maintained ~2–3 mmHg above baseline (El- Chami et al., 2017; Gerst III et al., 2011; Syed et al., 2013; Tester et al., 2014). The maintenance of carbon dioxide above baseline values is not primarily responsible for the initiation of long-term facilitation because control studies showed that the magnitude of sustained increases in ventilation were significantly less following exposure to sustained carbon dioxide compared to the magnitude of the response after exposure to intermittent hypoxia. Thus, it is established in humans that intermittent hypoxia must be accompanied by the maintenance of carbon dioxide at or above baseline levels to elicit ventilatory long-term facilitation. Since the initial discovery, published work has shown that long- term facilitation of ventilation can be initiated in healthy males and females (Babcock and Badr, 1998; Griffin et al., 2012; Harris et al., 2006; Lee et al., 2009; Pierchala et al., 2008; Stuckless et al., 2020; Syed et al., 2013; Vermeulen et al., 2020; Wadhwa et al., 2008), males and females with sleep apnea (Gerst III et al., 2011; Lee et al., 2009; Syed et al., 2013), and males and females with spinal cord injury (Sankari et al., 2015; Tester et al., 2014).
4.2. Mechanistic underpinnings of ventilatory long-term facilitation
Millhorn and colleagues originally demonstrated a ponto-medullary neural mechanism that elicits a long lasting (up to 90 min) increase in phrenic motor output following episodic carotid sinus nerve stimulation in anesthetized cats (Millhorn et al., 1980a; Millhorn et al., 1980b). Subsequently, numerous investigators demonstrated that intermittent hypoxia can elicit phrenic nerve long-term facilitation in rats characterized by a persistent increase in phrenic nerve burst amplitude lasting several hours after the final hypoxic episode (Bach and Mitchell, 1996; Hayashi et al., 1993). The role of carotid sinus nerve stimulation and intermittent hypoxia in inducing long-term facilitation indicates that the carotid bodies have a role in initiating this phenomenon.
In order to assess the influence of carotid body afferent discharge on long-term facilitation of ventilation following acute exposure to intermittent hypoxia in humans, Griffin and associates exposed twelve healthy male participants to hyperoxia (to prevent stimulation of carotid bodies) before and after exposure to intermittent hypoxia and sustained hypercapnia, as well as, before and after sustained hypercapnia (Griffin et al., 2012). The authors reported that hyperoxia-induced inhibition of carotid bodies did not affect the magnitude of ventilatory long-term facilitation indicating that long-term facilitation occurs independent of peripheral chemosensory plasticity in healthy humans (Griffin et al., 2012). This finding is similar to the results from rodent models which showed that ventilatory long-term facilitation was maintained despite inhibition of carotid body activity with hyperoxia after exposure to acute intermittent hypoxia (Baker and Mitchell, 2000; Xing et al., 2013). Considering their findings, Griffin and co-workers proposed that in addition to cellular pathways dependent on carotid body stimulation and ultimately the release of serotonin, lowering the pH of cerebrospinal spinal fluid in response to hypoxia and sustained hypercapnia might partially contribute to ventilatory long-term facilitation (Griffin et al., 2012). These authors also suggested that the time course of the phenomenon might reflect the course of recovery of hydrogen ion concentration. However, at the present time there is no evidence to support or refute this possibility. In contrast, Vermeulen and colleagues recently reported that ventilatory long-term facilitation was attenuated, but not abolished, following exposure to intermittent hypoxia (Vermeulen et al., 2020). This response occurred after peripheral chemoreceptor activity was reduced with supplemental oxygen during the recovery period (Vermeulen et al., 2020). This finding led Vermeulen and colleagues to suggest that the carotid bodies have a role in modulating ventilatory long-term facilitation.
The potential role of the peripheral chemoreceptors in initiating long- term facilitation might explain the enhanced magnitude of this phenomenon in individuals with obstructive sleep apnea. Intermittent hypoxia induced by apneas repetitively and chronically stimulate the carotid bodies in untreated patients with sleep apnea (Prabhakar, 2016). Chronic stimulation of the carotid bodies enhances the magnitude of sensory long- term facilitation (Prabhakar et al., 2009). Sensory long-term facilitation could be initiated by chronic nightly exposure to intermittent hypoxia and could lead to an overall increase in magnitude of ventilatory long- term facilitation in patients with obstructive sleep apnea. To test this hypothesis, our group exposed obstructive sleep apnea patients and healthy adults to twelve 4-min episodes of hypoxia (Lee et al., 2009). The results showed that the magnitude of ventilatory long-term facilitation was greater in individuals with obstructive sleep apnea compared to healthy controls during wakefulness (Lee et al., 2009).
The enhanced magnitude of carotid sensory long-term facilitation via exposure to chronic intermittent hypoxia might be modulated by increased oxidative stress and the generation of reactive oxygen species during re‑oxygenation similar to ischemia-reperfusion (Katayama et al., 2005; Pialoux et al., 2009). Likewise, oxidative stress and reactive oxygen species might also impact long-term facilitation independent of the peripheral chemoreceptors. More specifically, superoxide anions localized in the region of the phrenic motor nucleus are necessary for both induction and maintenance of phrenic long-term facilitation following acute intermittent hypoxia in anesthetized rats. This finding is based on the result that administration of a superoxide dismutase mimetic inhibits the expression of phrenic long-term facilitation (MacFarlane and Mitchell, 2008). Likewise, the administration of antioxidants in patients with obstructive sleep apnea reduced the magnitude of ventilatory long-term facilitation during recovery compared to placebo. This response could be the result of mitigating sensory long-term facilitation of the carotid bodies with the antioxidant treatment (Lee et al., 2009). However, the contribution of sensory long-term facilitation to respiratory long-term facilitation in obstructive sleep apnea is not completely understood and requires future study. In addition, long-term facilitation of ventilation in healthy males without sleep apnea was present and the magnitude unchanged after the administration of antioxidants (Lee et al., 2009). This finding indicates that other mechanisms, independent of accumulation of reactive oxygen species, contributes to the manifestation of long-term facilitation.
In addition to oxidative stress, inflammation is a common feature of many clinical diseases or disorders. Obstructive sleep apnea is viewed as a low-grade chronic inflammatory disease and is found to be associated with inflammatory markers including interleukin-6 and tumor necrosis factor α (Kheirandish-Gozal and Gozal, 2019). Beaudin et al., 2015, in their randomized cross-over study assessed the effect of inflammation on intermittent hypoxia-induced respiratory plasticity. This group administered two non-steroidal anti-inflammatory drugs in twelve healthy volunteers for 4 days prior to acute intermittent hypoxia exposure. The results showed that inflammation does not impact augmentation of minute ventilation following an acute intermittent hypoxia exposure in humans (Beaudin et al., 2015). However, their model of healthy humans exposed to intermittent hypoxia that matches the oxygen desaturation profile of obstructive sleep apnea is not an entirely accurate representation of the clinical condition as increased negative intrathoracic pressure, modulating carbon dioxide levels and sleep fragmentation associated with obstructive apneas were absent (Mateika, 2019).
Moreover, it is important to note that the magnitude of inflammatory signaling is impacted by the type of hypoxic stimulus (sustained vs intermittent) and the site of inflammation. Evidence from animal models suggests that inflammation in medullary respiratory centers contributes to chronic sustained hypoxia induced respiratory plasticity, whereas inflammation of the ventral horn of the spinal cord abolishes phrenic long-term facilitation induced by acute intermittent hypoxia (see review (Hocker et al., 2017) for more details). These models also provide evidence that systemic inflammation induces central nervous system inflammation by disrupting the integrity and permeability of the blood brain barrier (Liu et al., 2020). Although it is known that obstructive sleep apnea causes systemic inflammation, as evident by the inflammatory biomarkers in blood, there is no report of inflammation in the central nervous system in humans with this disorder. Given the scarcity of data and experiments conducted, it is presently difficult to comment on the definitive role of inflammation in respiratory plasticity, specifically ventilatory long-term facilitation, in healthy humans or humans with sleep apnea.
5. Upper airway long-term facilitation
Long-term facilitation of upper airway muscle activity, including the genioglossus and glossopharyngeal muscle, or the nerves that innervate the muscles, has been firmly established in rats and cats (Cao et al., 2010; ElMallah et al., 2016; Mateika and Fregosi, 1997; Mateika and Sandhu, 2011; Wilkerson et al., 2018). Long-term facilitation of upper airway muscle activity in humans could serve a beneficial purpose by increasing airway patency leading to the mitigation or elimination of apneic events in individuals with obstructive sleep apnea (Mateika et al., 2015; Mateika and Komnenov, 2017; Mateika and Narwani, 2009). Electromyographic recordings of genioglossus muscle activity from healthy individuals during wakefulness initially indicated that exposure to mild intermittent hypoxia does not initiate long-term facilitation of genioglossus muscle activity (Jordan et al., 2002). This response was absent in both males and females (Jordan et al., 2002). However, soon after, long-term facilitation of genioglossus muscle activity was initiated in healthy males during wakefulness (Harris et al., 2006) (Fig. 3). As with ventilatory long-term facilitation, this phenomenon was only evident if carbon dioxide levels were sustained above baseline (Harris et al., 2006). Along the same lines, work completed during sleep provided indirect evidence that intermittent hypoxia induced long-term facilitation of upper airway muscle activity was evident during sleep. Specifically, exposure to intermittent hypoxia resulted in sustained reductions in upper airway resistance in flow limited individuals coupled to long-term facilitation of ventilation (Babcock et al., 2003; Babcock and Badr, 1998). This response was not evident in non-flow limited individuals (Babcock et al., 2003; Babcock and Badr, 1998). The idea put forth was that long-term facilitation of the upper airway muscles improved airway patency and reduced resistance (Rowley et al., 2007; Shkoukani et al., 2002). This response alleviated flow limitation that ultimately led to sustained increases in ventilation (Rowley et al., 2007; Shkoukani et al., 2002). In support of this idea, Chowdhuri and colleagues directly showed that long-term facilitation of genioglossus muscle activity induced by mild intermittent hypoxia could be initiated in healthy humans during sleep (Chowdhuri et al., 2008). The magnitude of the response during sleep was similar in males and females and was greater (~ 2 times greater than baseline) (Chowdhuri et al., 2008) compared to wakefulness (~ 1.5 times greater than baseline) (Syed et al., 2013). However, despite the presence of genioglossus long-term facilitation, a sustained increase in ventilation was not evident (Chowdhuri et al., 2008). The authors proposed, based on work in animals, that activation of the upper airway muscles in individuals with a favorable upper airway anatomy might not lead to upper airway dilatation and decreased resistance (Chowdhuri et al., 2008). As a result, despite initiation of long-term facilitation of upper airway muscle activity increases in ventilation were not evident.
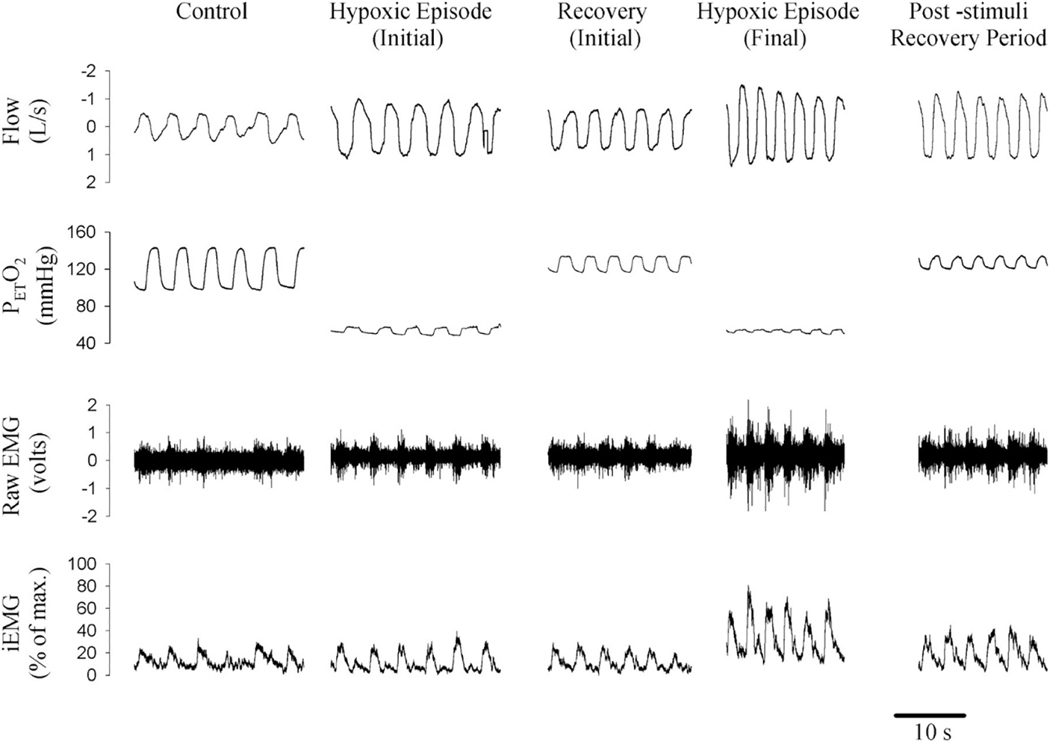
Fig. 3.
A raw figure obtained from a participant showing changes in peak genioglossus muscle activity from the initial hypoxic episode and subsequent recovery period to the final hypoxic episode and post-stimuli recovery period. Note that peak genioglossus muscle activity was greater during the last hypoxic episode compared with the initial episode and that genioglossus muscle activity during the post-stimuli recovery period was greater than measures obtained during baseline. This sustained increase in genioglossus muscle activity is referred to as long-term facilitation of upper airway muscle activity. Reprinted from “Long-term facilitation of ventilation and genioglossus muscle activity is evident in the presence of elevated levels of carbon dioxide in awake humans,” by D.P. Harris et al. Am. J. Physiol. Regul. Integr. Comp. Physiol. 291: 1111–1119, 2006.
Despite the hypothesis, along with supporting evidence, that the maintenance of carbon dioxide is required for the manifestation of long- term facilitation of upper airway muscle activity (Harris et al., 2006), more recent work seems to contradict the hypothesis at first glance (Deacon et al., 2017). Deacon and colleagues reported that long-term facilitation of genioglossus muscle activity was not evident following exposure to intermittent hypoxia and intermittent hypercapnia, even though carbon dioxide was maintained at baseline levels (Deacon et al., 2017). There are two possible reasons for these disparate findings. The first is that end-tidal measures of carbon dioxide may not exactly reflect arterial measures. Thus, because carbon dioxide was maintained at baseline levels and not slightly above, as was done in the investigation of Harris and colleagues (Harris et al., 2006), there is a possibility that arterial hypocapnia existed to some degree. The more likely possibility is that the manner in which carbon dioxide was administered could have a significant effect on the magnitude of long-term facilitation. In previous studies, carbon dioxide was sustained at or above baseline levels throughout the protocol (Chowdhuri et al., 2008; Harris et al., 2006). In other words, the same level of carbon dioxide was evident during both intermittent hypoxia episodes and recovery periods. In contrast, in Deacon and colleagues study, increases in carbon dioxide only occurred intermittently (Deacon et al., 2017). In other words, participants were exposed to both intermittent hypoxia and hypercapnia. Published findings have suggested that exposure to intermittent hypercapnia depresses phrenic nerve burst frequency in rats and consequently limits the expression of ventilatory long-term facilitation (Stipica et al., 2016; Valic et al., 2016). Thus, the use of intermittent hypoxia/hypercapnia could lead to the activation of two pathways, one originating from the locus coeruleus and the other from raphe nuclei. Investigations completed in animals indicate that activation of the locus coeruleus might inhibit the serotonergic pathway (de Carvalho et al., 2014). In contrast, the practice of maintaining carbon dioxide above the recruitment threshold during exposure to intermittent hypoxia ensures that hypocapnia is prevented and intermittent hypoxia initiated long-term facilitation is fully expressed [see review by (Mateika et al., 2018) for more details]. Therefore, the discrepancy in Deacon et al. findings compared to previous results might be explained by the variation in the application of carbon dioxide (intermittent vs. sustained) (Deacon et al., 2017).
The discovery that long-term facilitation of upper airway muscle activity could be initiated in humans was exciting because it presented the distinct possibility that initiation of this phenomenon could mitigate sleep apnea. However, as addressed below (see Progressive Augmentation, Long-Term Facilitation and Apnea Severity), exposure to therapeutic doses of intermittent hypoxia is accompanied by increases in the apnea/hypopnea index (Syed et al., 2013; Yokhana et al., 2012). The reason for this is that a concomitant enhancement of the ventilatory response to chemical stimuli (i.e. progressive augmentation of the hypoxic ventilatory response) negates the potential benefits of long-term facilitation of upper airway muscle activity (Mateika et al., 2015; Mateika and Komnenov, 2017; Mateika and Narwani, 2009).
5.1. Upper airway long-term facilitation & therapeutic continuous positive airway pressure
Based on this hypothesis, it was proposed that the beneficial impact of long-term facilitation of upper airway muscle activity would only manifest if the effects of an augmented ventilatory response were mitigated (Mateika and Komnenov, 2017). To address the hypothesis, the impact that an enhanced ventilatory response might have on apneic events was eliminated by treating participants with therapeutic continuous positive airway pressure to prevent flow limitation (El- Chami et al., 2017). At the same time, participants were exposed to 12 episodes of intermittent hypoxia (El-Chami et al., 2017). Following exposure to mild intermittent hypoxia, a reduction in upper airway resistance coupled to an increase in flow was evident at the therapeutic continuous positive airway pressure established at baseline (i.e. prior to exposure to mild intermittent hypoxia) (El-Chami et al., 2017). In contrast, following a sham protocol (i.e. exposure to compressed air for the duration of the mild intermittent hypoxia protocol) the airway resistance and flow were similar to baseline (El-Chami et al., 2017). After exposure to mild intermittent hypoxia, the positive airway pressure was also reduced by 5–7 cmH2O in a step-wise fashion (El-Chami et al., 2017). The results showed that airway resistance and airflow were maintained at baseline levels despite the decrease in pressure (El-Chami et al., 2017). In contrast, when the pressure was decreased following the sham protocol, airway resistance increased, and flow decreased compared to baseline (El-Chami et al., 2017). Long-term facilitation of upper airway muscle activity may have been initiated for these outcomes to be observed.
Although the impact of long-term facilitation of upper airway muscle activity apparently does not manifest under natural conditions, a positive outcome (i.e. reductions in continuous positive airway pressure) following initiation of long-term facilitation of upper airway muscle activity was revealed (El-Chami et al., 2017), when the potential detrimental effects of an enhanced ventilatory response to chemical stimuli were mitigated. This outcome measure could ultimately be a benefit for two primary reasons. The first is that reductions in pressure might improve adherence with continuous positive airway pressure (Mateika and Komnenov, 2017). This premise is supported by work showing that individuals using both an oral compliance along with continuous positive airway pressure, results in reductions in continuous positive airway pressure coupled to improved treatment adherence (Liu et al., 2017). In addition, it was proposed that improvement in some co- morbidities would exceed the response expected by the improved adherence (Mateika and Komnenov, 2017). It was suggested this was the case because mild intermittent hypoxia has direct beneficial effects on a number of physiological systems (Mateika and Komnenov, 2017).
Thus, it was investigated if repeated daily exposure to mild intermittent hypoxia leads to long-term facilitation of upper airway muscle activity, reduced therapeutic continuous positive airway pressure and improved adherence. This improved adherence could lead to greater mitigation of cardiovascular co-morbidities. Thus, individuals with obstructive sleep apnea were exposed daily to twelve 2-min episodes of hypoxia for 5 days/week over a 3-week period (Panza et al., 2020a, 2020b). Another group of individuals with obstructive sleep apnea was also exposed to a sham protocol (i.e. twelve 2-min episodes of compressed air for 5 days/week over a 3-week period) (Panza et al., 2020a, 2020b). Blood pressure was measured over a 24-h time period before and after the treatment period (Panza et al., 2020a). In addition, the critical closing pressure, therapeutic continuous positive airway pressure, and at-home adherence to continuous positive airway pressure before and after 3 weeks of treatment with mild intermittent hypoxia were also determined (Panza et al., 2020b). The results, published to date in abstract form, revealed that the critical closing pressure following 3 weeks of mild intermittent hypoxia was less positive and this was coupled to a reduction in therapeutic continuous positive airway pressure (Panza et al., 2020b). These findings, along with the previous results, suggest that long-term facilitation of upper airway muscle activity was likely responsible for the modification in the critical closing pressure and therapeutic continuous positive airway pressure (El-Chami et al., 2017;Panza et al., 2020b). In conjunction with these findings, nightly adherence with continuous positive airway pressure significantly improved (Panza et al., 2020b). It is suggested that the improved adherence was due in part to the reduction in continuous positive airway pressure (El-Chami et al., 2017; Panza et al., 2020b). Modifications in the arousal threshold also likely contributed to improved adherence (Alex et al., 2019). The improved adherence combined with the direct influence of intermittent hypoxia led to dramatic reductions in blood pressure not typically seen after 3 weeks of treatment with continuous positive airway pressure (Panza et al., 2020a).
6. Factors that impact the magnitude of respiratory plasticity
6.1. Sex
To our knowledge, only three studies have examined the impact of sex on progressive augmentation. In two studies, progressive augmentation was evident during exposure to intermittent hypoxia in males and females during wakefulness (Syed et al., 2013; Wadhwa et al., 2008) but was not evident in either sex during sleep (Syed et al., 2013) (see Wake vs. Sleep for a discussion on the impact of arousal state on progressive augmentation). Although evident during wakefulness, the degree of progressive augmentation was similar between males and females (Syed et al., 2013; Wadhwa et al., 2008). In contrast, following exposure to intermittent hypoxia the ventilatory response to hypoxia and hypercapnia was greater in males compared to females (Morelli et al., 2004). Additional work is required to decipher the reason for the discrepant findings. Perhaps the issues associated with quantifying the hypoxic ventilatory response during exposure to intermittent hypoxia make it difficult to detect sex differences (see Manifestation of Progressive Augmentation for additional discussion). On the other hand, participants in the studies designed to explore sex differences in progressive augmentation were studied in the follicular phase of their menstrual cycle (Morelli et al., 2004; Syed et al., 2013; Wadhwa et al., 2008). Thus, additional work is required to determine if hormone fluctuations in the luteal phase of the menstrual cycle will reveal any differences. However, given findings that have shown that sex hormones are linked to differences in the ventilatory response to modifications in oxygen or carbon dioxide levels (Lozo et al., 2017; Zhou et al., 2000), sex differences in progressive augmentation might be anticipated. If this is the case, the increase in breathing events that have been documented across the night might be greater in males compared to females (Lozo et al., 2017) (see Progressive Augmentation, Long-Term Facilitation and Apnea Severity).
Like progressive augmentation, the magnitude of ventilatory long- term facilitation could also be impacted by sex differences. Administration of testosterone restores the expression of ventilatory long-term facilitation in gonadectomized male rats (Zabka et al., 2006). Similarly, the expression of ventilatory long-term facilitation in the proestrus phase of the estrus cycle in freely breathing female rats (McIntosh and Dougherty, 2019), and the restoration of ventilatory long-term facilitation following estrogen supplementation in ovariectomized female rats (Dougherty et al., 2017) indicates that estrogen signaling has a role in the initiation of respiratory plasticity. Taken together, male and female sex hormones impact the magnitude of long-term facilitation in rats. Therefore, the manifestation or the amplitude of respiratory motor plasticity in response to acute intermittent hypoxia exposure could be different in human males compared to females.
However, in contrast to the findings from animal experiments, it was found that the magnitude of ventilatory long-term facilitation was similar in healthy males and females (Syed et al., 2013; Wadhwa et al., 2008), as well as, in men and women with obstructive sleep apnea (Syed et al., 2013). Recently, Vermeulen and colleagues also reported that the amplitude of long-term facilitation following intermittent hypercapnic hypoxia was similar in healthy men and women (Vermeulen et al., 2020). These investigators suggested that the phase of the menstrual cycle (i.e. early follicular phase when the estrogen level is not at its peak) could have limited the positive influence of sex hormone on minute ventilation (Vermeulen et al., 2020). Future work studying the manifestation of long-term facilitation across different stages of the menstrual cycle is needed to fully understand the impact of sex hormones on respiratory neural plasticity in humans. In contrast to the findings in healthy men and women and those with sleep apnea, Tester et al. (2014) reported that men with chronic incomplete spinal cord injury have a greater increase in ventilatory long-term facilitation than women following repeated daily exposure to intermittent hypoxia, although the sample size was limited (Tester et al., 2014). Post-injury alterations in the levels of testosterone in men (Bauman et al., 2014) and estrogen in women (Lombardi et al., 2007) have been reported, indicating that sex hormones could impact on the magnitude of long- term facilitation. However, serum levels of sex steroid hormones were not measured in (Tester et al., 2014) investigation. Consequently, the impact of sex hormones on the differential expression of ventilatory long-term facilitation in men and women with spinal cord injury remains to be determined.
6.2. Age
The effects of aging on the control of breathing are reflected in the modifications to several neural, morphological and cellular biomarkers. These modifications include reductions in (i) the number of phrenic motor neurons (Elliott et al., 2016; Fogarty et al., 2018) (ii) phrenic motor neuron dendritic volume (Fogarty et al., 2018) (iii) brain-derived neurotrophic factor expression in phrenic motoneurons (Greising et al., 2015) (iv) serotonergic innervation of spinal segments in contact with the phrenic motor nucleus (Ko et al., 1997) (v) the neurotrophic influence on diaphragmatic neuregulin (Bordoni et al., 2020) (vi) transdiaphragmatic pressure (Khurram et al., 2018; Polkey et al., 1997; Tolep et al., 1995) and (vii) reductions in the force generating capacity of the diaphragm (Greising et al., 2013; Khurram et al., 2018). In addition, aging is also linked to increases in phrenic nerve latency (Behan et al., 2002; Bordoni et al., 2020; Elliott et al., 2016; Shah et al., 2019). These modifications suggest that age could have a profound impact on the manifestation and/or magnitude of respiratory motor plasticity including ventilatory long-term facilitation (see Mechanistic underpinnings of long-term facilitation for more details) Indeed, Zabka et al. (2001a) showed that the magnitude of long-term facilitation following isocapnic intermittent hypoxia in aged male rats was reduced compared to young rats (Zabka et al., 2001a; Zabka et al., 2001b). These authors speculated that an age-related deterioration of serotonergic modulation of respiratory motor output was responsible for the reduction.
Chowdhuri and colleagues demonstrated in humans that aging is linked to a reduction in the magnitude of long-term facilitation during non-rapid eye movement sleep since long-term facilitation was found in young but not older men (Chowdhuri et al., 2015; Chowdhuri et al., 2010). The authors suggested that the absence of ventilatory long-term facilitation in older adults during sleep could be due to an age-dependent decline in raphe neuronal activity, reduced availability of serotonin, or an age-related decrease in serum testosterone levels in older men (Chowdhuri et al., 2015; Chowdhuri et al., 2010). However, their study was not designed to tease out the relative contribution of age vs. reduction in sex hormone levels on the magnitude of ventilatory long-term facilitation.
6.3. Wake vs. Sleep
Arousal state could play a role in the manifestation of respiratory plasticity. It was found, in both males and females, that progressive augmentation was not evident during sleep but was evident during wakefulness (Syed et al., 2013) (Fig. 4). This result was evident in both healthy males and females, and in males and females with sleep apnea that were treated with continuous positive airway pressure during sleep (Syed et al., 2013). The absence of progressive augmentation during sleep has also been replicated in healthy older and younger males and females (Chowdhuri et al., 2015). The absence of progressive augmentation during sleep relative to wakefulness does not exclude this phenomenon from being initiated during sleep. As stated above, progressive augmentation was not observed in rats following exposure to an intermittent hypoxia protocol of moderate intensity but was observed following exposure to a more severe intermittent hypoxia protocol (Nichols et al., 2012). The intermittent hypoxia protocol used in Syed et al. study was of moderate severity (Syed et al., 2013). Thus, it is reasonable to speculate that a more intense protocol may have resulted in the manifestation of progressive augmentation during sleep (as it did in anesthetized rats), while a less severe stimulus was required for the initiation of these phenomenon during wakefulness.
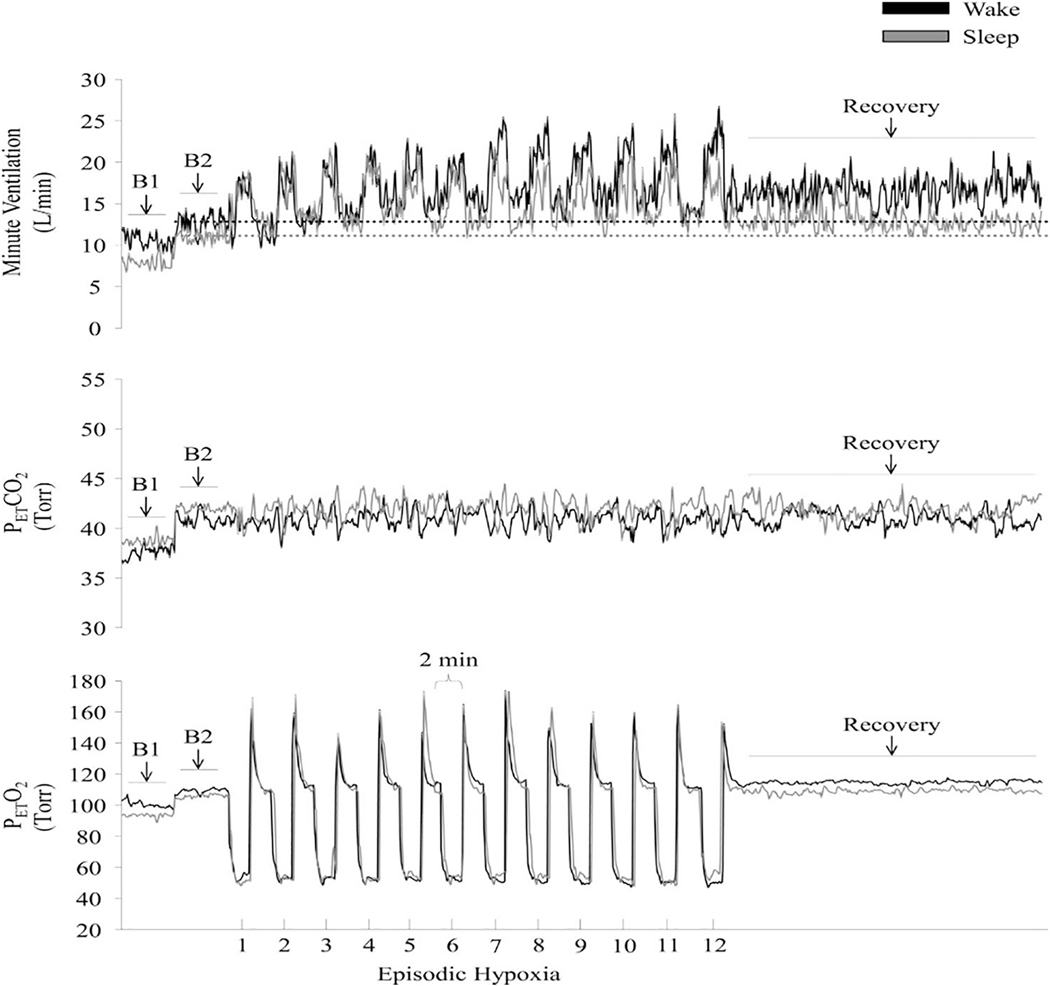
Fig. 4.
Measures of minute ventilation (top), PETCO2 (middle), and PETO2 (bottom) during completion of an intermittent hypoxia protocol during wakefulness and sleep. A raw record of breath-by-breath minute ventilation recorded from one participant exposed to intermittent hypoxia is shown. The dotted lines represent baseline values specific to each state. Note that minute ventilation during the end-recovery period was greater than baseline during wakefulness as well as during sleep. Also note that the magnitude of the increase during recovery compared to baseline was greater during wakefulness compared to sleep. B1, last 5 min of the initial normocapnic 10 min baseline period; B2, last 5 min of the second baseline period during which PETCO2 was elevated 3 Torr above B1 measures. Reprinted from “The impact of arousal state, sex, and sleep apnea on the magnitude of progressive augmentation and ventilatory long-term facilitation” by Z. Syed et al. J. Appl. Physiol. 114: 52–65, 2013.
The impact of arousal state (wake vs. sleep) on the magnitude of ventilatory long-term facilitation is also important since this phenomenon could have a role in improving ventilatory and upper airway stability during sleep (see Upper Airway Long-Term Facilitation for details). Intermittent hypoxia may elicit ventilatory long-term facilitation during sleep by preferentially recruiting upper airway dilator muscles (Chowdhuri et al., 2008; El-Chami et al., 2017). In contrast, Deacon and colleagues did not observe ventilatory long-term facilitation following exposure to intermittent hypoxia/hypercapnia during non-rapid eye movement sleep in healthy males (Deacon et al., 2017). The absence of long-term facilitation in Deacon et al. (2017) investigation could be linked to exposure to intermittent hypercapnia as outlined above (see Upper Airway Long-Term Facilitation). On the other hand, recent studies have reported that long-term facilitation of ventilation was initiated in healthy humans following exposure to an intermittent hypoxia/hypercapnia protocol (Ott et al., 2020; Vermeulen et al., 2020) similar to the one employed by Deacon and colleagues (Deacon et al., 2017). The primary difference between the investigations is that the participants in Deacon et al. (Deacon et al., 2017) study were exposed to intermittent hypoxia/hypercapnia during sleep, while participants in subsequent investigations were exposed to this stimulus during wakefulness (Ott et al., 2020; Vermeulen et al., 2020). Thus, arousal state might have a profound influence on the magnitude of long-term facilitation.
This suggestion is supported by the work from Syed and co-workers that systematically examined the impact of arousal state on the magnitude of ventilatory long-term facilitation in humans. Healthy adults and adults with obstructive sleep apnea were exposed to intermittent hypoxia during both wakefulness and sleep (Syed et al., 2013). The amplitude of ventilatory long-term facilitation in individuals with obstructive sleep apnea was reported to be greater compared to healthy adults. It was suggested that chronic nightly exposure to intermittent hypoxia, a hallmark of sleep apnea, could be responsible for the increased amplitude of ventilatory long-term facilitation in obstructive sleep apnea patients. In addition, the results showed that the amplitude of ventilatory long-term facilitation was greater during wakefulness compared to sleep in both healthy adults and individuals with sleep apnea (Syed et al., 2013) (Fig. 4). These findings were contrary to a hypothesis developed from animal studies which suggested that the magnitude of long-term facilitation would be greater during sleep compared to wakefulness.
The difference in magnitude of progressive augmentation and long- term facilitation between arousal states could reflect the adjustment of neuromodulator(s) between wakefulness and sleep. Serotonin would be an obvious possibility, since its levels vary between wake and sleep (España and Scammell, 2011). Likewise, serotonin plays an important role in the initiation of ventilatory long-term facilitation (Bach and Mitchell, 1996). Alternatively, its impact on the magnitude of progressive augmentation in anesthetized rats and cats (Fregosi and Mitchell, 1994; Nichols et al., 2012) is less certain. Independent of the specific neuromodulator, this hypothesis relies on the premise that basal discharge rates of neurons that release a given neuromodulator during wakefulness are closer to maximal levels (España and Scammell, 2011). If so, there would be a limited capacity to increase activity during and following administration of intermittent hypoxia. Consequently, the magnitude of progressive augmentation and long-term facilitation could be marginal under these circumstances. Conversely, during sleep the basal discharge rate of these neurons might be diminished. Accordingly, there might be room for a greater range of activity during and after administration of intermittent hypoxia, before maximum rates are achieved. As a result, the magnitude of progressive augmentation and long-term facilitation might be greater during sleep compared with wakefulness. However, this speculation is not supported by studies in humans during sleep, which reported that progressive augmentation is not elicited during this arousal state (Chowdhuri et al., 2015; Chowdhuri et al., 2010; Syed et al., 2013). Likewise, the magnitude of ventilatory long-term facilitation is reduced during sleep compared to wakefulness. As stated above, the differences observed between arousal states could be related to the intensity of the hypoxic stimulus. However, equally plausible is that the absence of progressive augmentation and reductions in the magnitude of long-term facilitation during sleep compared to wake are not principally dependent on modifications in the dynamic range of neuromodulators in humans.
The increase in magnitude of long-term facilitation during wakefulness might be related to the interaction between two cellular pathways (Gs and Gq) that are involved in the initiation of long-term facilitation (Dale-Nagle et al., 2010; Pamenter and Powell, 2013). The Gs and Gq are known to exhibit crosstalk inhibition, whereby activation of the Gs pathway diminishes the magnitude of long-term facilitation mediated initially by the Gq pathway (Nichols et al., 2012). Adenosine initiates ventilatory long-term facilitation via activation of the Gs pathway (via A2 receptors), while serotonin activates the Gq pathway (via 5HT2 receptors) following intermittent hypoxia (Dale-Nagle et al., 2010). Adenosine is a metabolic by-product that progressively increases during wakefulness and gradually declines, reaching its lowest concentration, during sleep (Brown et al., 2012). In the study by Syed and colleagues, intermittent hypoxia was administered to participants soon after waking from sleep in early morning (Syed et al., 2013). Thus, it possible that lower levels of adenosine weakened the crosstalk inhibition of the Gq pathway that resulted in an increased amplitude of ventilatory long-term facilitation during wakefulness. Lastly, orexin facilitates serotonergic neurons located in the raphe nuclei (Berthoud et al., 2005; Yang et al., 2019) and thus may have also contributed to the enhanced magnitude of ventilatory long-term facilitation observed during wakefulness in the study by (Syed et al., 2013). Hence, the magnitude of ventilatory long-term facilitation may be influenced by the interaction of various neuromodulators that include, but are not limited to, serotonin, adenosine, and orexin.
6.4. Repeated daily exposure
Repeated daily exposure to a mild intermittent hypoxia protocol of similar intensity, over consecutive days, might impact the magnitude of progressive augmentation. The repeated daily exposure could occur naturally because of obstructive sleep apnea or as the result of experimentally administered intermittent hypoxia over a selected number of consecutive days (Mateika and Narwani, 2009). If natural exposure to intermittent hypoxia enhances progressive augmentation, one might anticipate an enhanced level of progressive augmentation in individuals with obstructive sleep apnea compared to healthy individuals during or immediately following a one-time acute exposure to intermittent hypoxia. Indeed, it has been shown that the ventilatory response progressively increases from the initial to the final episode of an intermittent hypoxia protocol in both healthy humans and humans with obstructive sleep apnea (Lee et al., 2009). However, the ventilatory response during the final hypoxic episode of the protocol was greater in individuals with obstructive sleep apnea compared to matched healthy controls (Lee et al., 2009). Unfortunately, baseline measures prior to each episode also gradually increased so it was difficult to quantify the hypoxic ventilatory response (see Manifestation of Progressive Augmentation for further discussion). No matter, this issue was eliminated when measures of the ventilatory response to hypoxia using a rebreathing method was employed following exposure to intermittent hypoxia. The results showed that the ventilatory response to hypoxia was greater in individuals with obstructive sleep apnea compared to control, after accounting for the impact of carbon dioxide (Lee et al., 2009). Given that the obstructive sleep apnea and control groups were matched for several anthropometric variables, and the exposure to chemical stimuli (hypoxia and hypercapnia) was similar between the groups it is believed that the nightly chronic exposure to intermittent hypoxia was responsible for the enhanced progressive augmentation.
If this hypothesis is correct, the ventilatory response to a brief hypoxic exposure (e.g. 3 min) might be enhanced in individuals with sleep apnea, because this population is exposed to intermittent hypoxia on a nightly basis. Some studies have indicated that the ventilatory response to a brief hypoxic exposure is enhanced in obstructive sleep apnea participants (Narkiewicz et al., 1999; Tun et al., 2000), while others have reported the opposite finding (i.e. the hypoxic response is depressed) (Lin, 1994; Osanai et al., 1999). Reasons for the divergent findings have not been fully explained. However, it was hypothesized previously (Mateika and Narwani, 2009) that chronic exposure to mild hypoxia might enhance the acute hypoxic response, while chronic exposure to severe hypoxia might blunt the response (Mateika and Narwani, 2009), in a manner observed in individuals exposed to hypoxia at high altitude for short (i.e. days) or long periods of time (i.e. years) (Weil, 1986). Thus, factors such as age (i.e. duration of exposure to hypoxia), apnea/hypopnea index (i.e. frequency of hypoxic exposure) and degree of oxygen desaturation at night (i.e. intensity of hypoxemia) might impact on the acute response measured in individuals with obstructive sleep apnea. If this is the case, enhancement of the ventilatory sensitivity to hypoxia might be particularly evident in young individuals who experience mild-to-moderate forms of apnea (Mateika and Narwani, 2009). Conversely, exposure to chronic intermittent hypoxia might blunt these responses in individuals who are older and experience severe hypoxemia throughout a given night (Mateika and Narwani, 2009).
This belief was initially supported by studies in animals which showed that repeated daily exposure to intermittent hypoxia leads to enhanced progressive augmentation of ventilation in awake cats or phrenic nerve activity in anesthetized rats (Ling et al., 2001; McGuire et al., 2003; Pawar et al., 2008; Rey et al., 2004). These findings were subsequently reinforced by unequivocal studies in healthy humans which showed that exposure to a small or large number of hypoxic episodes of short duration, each day for a duration ranging from 4 days to two weeks, leads to enhancement of the hypoxic ventilatory response (Ainslie et al., 2007; Foster et al., 2005; Gilmartin et al., 2008; Koehle et al., 2007). Likewise, it was shown that exposure to intermittent hypoxia over a 10-day time period led to an enhanced hypoxic ventilatory response on the last compared to the initial day of exposure in individuals with obstructive sleep apnea (Gerst III et al., 2011). As an aside, repeated daily exposure to sustained hypoxia more often than not also initiates a similar increase in the hypoxic ventilatory response following exposure (Garcia et al., 2000; Katayama et al., 2001; Lusina et al., 2006). Whether a similar mechanism is responsible for comparable outcome measures, despite differences in protocol variables, is presently unknown.
Similar to progressive augmentation, repeated daily exposure to isocapnic intermittent hypoxia for 12 h a day over 7 consecutive days increased the magnitude, as well as, the duration of ventilatory long- term facilitation in spontaneously breathing awake rats. This increase persisted for at least 3 days after exposure to intermittent hypoxia (McGuire et al., 2003). This initial finding in rats supported the contention that the magnitude of ventilatory long-term facilitation in humans may be enhanced following repeated daily exposure to intermittent hypoxia. Thus, the effect of repeated daily exposure on the magnitude of ventilatory long-term facilitation was investigated in sleep apnea patients following exposure to intermittent hypoxia each day for 10-days (Gerst III et al., 2011). Results showed that the magnitude of ventilatory long-term facilitation was greater on day 10 vs. day 1 (Fig. 5). No difference was evident in minute ventilation and its components in sleep apnea patients following a sham protocol (i.e. exposure to room air) (Fig. 5). It was proposed that the enhanced amplitude of ventilatory long-term facilitation supported the notion that repeated nighttime exposure to intermittent hypoxia experienced by the patients with sleep apnea enhances the magnitude of long-term facilitation (Gerst III et al., 2011). This suggestion was supported by other findings, which showed that the magnitude of long-term facilitation was greater in sleep apnea patients compared to healthy individuals following an acute exposure to intermittent hypoxia (Lee et al., 2009; Syed et al., 2013).
Fig. 5.
Measures of minute ventilation during completion of an intermittent hypoxia or sham protocol on the first and tenth day of exposure to the protocol. A raw record of breath-by-breath minute ventilation recorded from one participant exposed to intermittent hypoxia (left panels) and from one participant exposed to compressed air (sham; right panels) on the first and tenth day of exposure. Note that minute ventilation during baseline and end recovery were greater on the final compared with the initial day of the intermittent hypoxia protocol. This increase indicates that repeated daily exposure enhances the magnitude of ventilatory long- term facilitation. Also note that measures of minute ventilation were similar on the initial and final days of the sham protocol. Reprinted from “The hypoxic ventilatory response and ventilatory long-term facilitation are altered by time of day and repeated daily exposure to intermittent hypoxia” by D.G. Gerst III et al. J. Appl. Physiol. 110: 15–28, 2011.
Results from other studies have also provided indirect evidence that repeated exposure to intermittent hypoxia enhances the expression of ventilatory long-term facilitation. Foster and colleagues showed that repeated daily exposure to isocapnic hypoxic episodes for 12 days in healthy humans resulted in an increase in baseline breathing frequency (with no difference in minute ventilation and tidal volume) on the final compared to the initial days of the protocol (Foster et al., 2005). The increase in baseline breathing frequency could be a marker of ventilatory long-term facilitation. However, the expression of ventilatory long- term facilitation may have been limited because carbon dioxide was not maintained above baseline levels (see Manifestation of ventilatory long- term facilitation for additional details). Also note that repeated daily exposure to intermittent hypoxia did not initiate increases in ventilation and/or its components in a couple of investigations (Ainslie et al., 2007; Chacaroun et al., 2016). In at least one investigation, the lack of response may have occurred because repeated daily exposure to a few brief episodes of hypoxia was only administered over 3 days. Likewise, in both studies carbon dioxide was not sustained above baseline values in contrast to the study by Gerst and co-workers (Gerst III et al., 2011).
6.5. Timing of application
In addition to the repeated application of intermittent hypoxia, the timing of the application might also impact on the magnitude of progressive augmentation and the potential detrimental outcome measures linked to this phenomenon (see Progressive Augmentation, Long-Term Facilitation and Apnea Severity). Assuming that the number, duration and intensity of hypoxic episodes are similar, the extent to which the hypoxic ventilatory response progressively increases in response to intermittent hypoxia may be greatest in the early evening compared to other times of the day. This may be the case because the hypoxic ventilatory response is lower in the evening compared to other times of the day, at least in individuals with obstructive sleep apnea (Gerst III et al., 2011). In support of this notion, it has been shown that chemoreflex sensitivity and loop gain (which is impacted in part by modifications in chemoreflex sensitivity) during sleep is lower in the evening compared to the morning and afternoon in individuals with obstructive sleep apnea (El-Chami et al., 2014; Puri et al., 2020). Given these findings, there might be a greater range available for the hypoxic ventilatory response to increase during exposure to intermittent hypoxia. On the other hand, given that the absolute hypoxic ventilatory response is greater in the morning (Gerst III et al., 2011), the capacity to increase in magnitude could be reduced compared to modifications in the evening. The modulation in absolute chemoreflex sensitivity might be mediated by a circadian variation in one or more neuromodulators. The most attractive possibility is melatonin, because it has been shown to enhance the response of the carotid bodies to hypoxia in rats (Chen et al., 2005; Tjong et al., 2006) and to vary in a diurnal fashion (Brown et al., 1997). This suggestion is supported by the work of Hernandez and colleagues who showed that melatonin peaked in the evening and declined thereafter in healthy individuals (Hernandez et al., 2007). In contrast, melatonin was found to gradually increase in individuals with obstructive sleep apnea, so that peak levels were recorded in the morning compared with the evening (Hernandez et al., 2007). This finding supports our hypothesis that the increase in the hypoxic ventilatory response observed in the morning compared with the evening might be mediated by melatonin. Whether or not this neuromodulator or a different modulator is responsible for the proposed variation in progressive augmentation between the evening and morning requires further investigation.
Independent of the impact that circadian rhythmicity might have on the magnitude of progressive augmentation, the time at which intermittent hypoxia is administered will likely have an influence on potential outcome measures. If intermittent hypoxia is administered immediately prior to sleep or perhaps during sleep, the detrimental effects of progressive augmentation are likely to have their greatest impact (Syed et al., 2013; Yokhana et al., 2012) (see Progressive Augmentation, Long-Term Facilitation and Apnea Severity). If intermittent hypoxia is applied many hours prior to sleep (e.g. in the morning) any potential effects linked to progressive augmentation might be mitigated. On the other hand, potential beneficial effects that might serve to mitigate sleep apnea could also be minimized.
Furthermore, it has been reported that ventilatory long-term facilitation in obstructive sleep apnea patients during wakefulness was greater in the evening compared to the morning on the first and tenth day of exposure to intermittent hypoxia (Gerst III et al., 2011). Moreover, the difference in magnitude was augmented by repeated daily exposure to intermittent hypoxia (Gerst III et al., 2011). Also, the magnitude of ventilatory long-term facilitation during sleep in patients with obstructive sleep apnea was greater in the evening compared to the morning (El-Chami et al., 2017). Mechanistically, the diurnal variation in long-term facilitation could be secondary to the day-night variation in the levels of serotonin or brain-derived neurotrophic factor (Cain et al., 2017). Specifically, the acrophase of serotonin is reported to be just before the onset of the dark period (Cain et al., 2017). In contrast, a decreased synthesis and release is found in the early morning (Sun et al., 2002). Likewise, brain-derived neurotrophic factor exhibits a 12-h circadian rhythm with peaks occurring at the second half of the subjective day and night (Coria-Lucero et al., 2016). Thus, circadian alterations in the levels of these neuromodulators may impact the magnitude of ventilatory long-term facilitation. However, further human studies are required to support this speculation, which is based on mechanistic findings from animal models.
6.6. Intermittent vs. sustained hypoxia
The profile of the hypoxic stimulus plays an important role in the expression of respiratory motor plasticity. Long-term facilitation of phrenic nerve activity is more robust after exposure to acute intermittent hypoxia compared to acute sustained hypoxia of a similar intensity and cumulative duration, in awake goats (Dwinell et al., 1997) and rats (Baker and Mitchell, 2000). Investigators initially hypothesized, with supporting evidence, that intermittent hypoxia induced long-term facilitation is predominantly a central neural phenomenon mainly dependent on serotonergic neuromodulation of the respiratory network including medullary respiratory neurons and phrenic motor neurons (Mitchell et al., 2001a). In contrast, sustained hypoxia elicits ventilatory acclimatization, which is a time-dependent phenomenon initiated by a cascade of cellular events in the carotid body that leads to an increase in receptor sensitivity (Bisgard, 2000).
Several completed studies explored the impact of pattern sensitivity (sustained vs. intermittent hypoxia) on the magnitude of respiratory plasticity in humans. The preponderance of evidence from data collected over the last 3 decades indicates that intermittent hypoxia initiates ventilatory long-term facilitation in humans (see section Manifestation of ventilatory long-term facilitation). On the other hand, many investigations have shown that exposure to sustained isocapnic hypoxia in either males or females does not elicit long-term facilitation of ventilation (McEvoy et al., 1996; Querido et al., 2010; Tamisier et al., 2005; Xie et al., 2001). Instead, termination of sustained hypoxia is typically accompanied by the immediate return of ventilation to baseline levels.
Despite these latter findings, Griffin and coworkers reported that a sustained increase in minute ventilation was evident following exposure to both acute intermittent hypoxia and sustained hypoxia in awake healthy humans, when the partial pressure of carbon dioxide was sustained 4–5 mmHg above baseline levels (Griffin et al., 2012). The mechanistic explanation for the similar response remains to be determined. One possibility is that the activation of two different cellular pathways led to a similar outcome. The initiation of phrenic motor facilitation (that includes phrenic long-term-facilitation) may be mediated by competing cellular pathways. One pathway is mediated by adenosine 2A receptors and the other via serotonin 5HT-2 receptors. Activation of a given pathway might be dependent on the pattern and severity of hypoxia (Devinney et al., 2016). Specifically, severe acute sustained hypoxia (SaO2 <80%) may elicit phrenic long-term facilitation via adenosine receptor activation while serotonin receptor activation mediates moderate acute intermittent hypoxia induced phrenic long-term facilitation (see section Mechanistic underpinnings of ventilatory long-term facilitation for details). Thus, the initiation of long-term facilitation following sustained hypoxia in Griffin et al. investigation compared to others could be linked to the intensity of hypoxia employed (Griffin et al., 2012). However, oxygen saturation levels achieved were not reported making it difficult to ascertain the intensity of the hypoxic stimulus (moderate or severe) used in the protocol.
7. Progressive augmentation, long-term facilitation & apnea severity
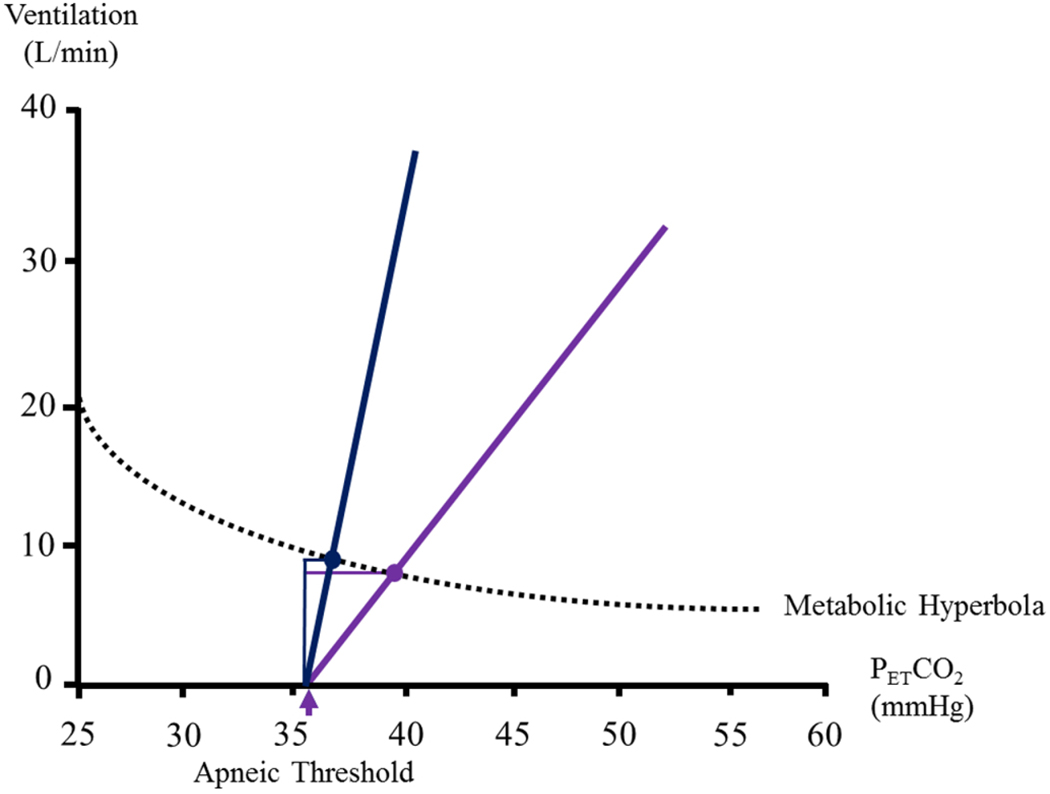
It is uncertain if ventilatory long-term facilitation and long-term facilitation of upper airway muscle activity is manifested spontaneously in obstructive sleep apnea patients who are exposed to daily night- time intermittent hypoxia. The reason this form of plasticity might not be evident is that carbon dioxide levels (i.e. hypocapnia) are often reduced (Mateika et al., 2018), and the maintenance of carbon dioxide at normocapnic levels is necessary for the manifestation of long-term facilitation in humans (Harris et al., 2006; Mateika et al., 2018). When humans are exposed to intermittent hypoxia during sleep, ventilatory long-term facilitation may be initiated (Chowdhuri et al., 2008; Syed et al., 2013). However, the increase in ventilation might contribute to the reduction in the partial pressure of carbon dioxide, causing a subsequent reduction in minute ventilation, thereby limiting the expression of long-term facilitation (Deacon et al., 2018; Mateika et al., 2015; Mateika and Komnenov, 2017; Mateika and Narwani, 2009; Mateika and Syed, 2013). More specifically, ventilatory long-term facilitation causes a leftward increase along the metabolic hyperbola curve (Deacon et al., 2018) (Fig. 6). This leftward increase induced by long-term facilitation, is coupled to another form of respiratory plasticity (i.e. progressive augmentation) (Mateika et al., 2015; Mateika and Komnenov, 2017; Mateika and Syed, 2013), which is responsible for the increased ventilatory response to hypoxia and/or hypercapnia (Fig. 6). As the apneic threshold in humans is not altered by intermittent hypoxia (Chowdhuri et al., 2010), the leftward increase along the metabolic hyperbola, coupled with the increased sensitivity to hypoxia and/or hypercapnia, narrows the carbon dioxide reserve (which is the difference between the partial pressure of carbon dioxide that corresponds to breathing at rest and the partial pressure of carbon dioxide that demarcates the apneic threshold) (Chowdhuri et al., 2010) (Fig. 6). The increased ventilatory response to hypoxia/hypercapnia coupled to a reduced carbon dioxide reserve promotes an increase in apneic events during non-rapid eye movement sleep by forcing carbon dioxide levels below the apneic threshold.
Fig. 6.
A schematic diagram showing the potential changes in minute ventilation, chemoreflex sensitivity and the carbon dioxide reserve that might occur as a consequence of exposure to intermittent hypoxia. Note that exposure to intermittent hypoxia will increase minute ventilation (purple circle represents ventilation before intermittent hypoxia and blue circle represents minute ventilation after intermittent hypoxia on the line labelled metabolic hyperbola). Likewise, chemoreflex sensitivity is enhanced after (blue line) compared to before (purple line) exposure to intermittent hypoxia. As a result, the carbon dioxide reserve (the difference between resting values of the partial pressure of carbon dioxide and the carbon dioxide value that demarcates the apneic threshold) is reduced after (blue horizontal line) compared to before intermittent hypoxia (purple horizontal line). The consequence of these modifications may lead to the perpetuation of apneic events. The purple arrow indicates the apneic threshold.
To investigate the impact of intermittent hypoxia on apnea severity, patients with obstructive sleep apnea were exposed to twelve 4-min hypoxic episodes while carbon dioxide was sustained 3 Torr above baseline (Yokhana et al., 2012). A second group of patients with obstructive sleep apnea were exposed to a sham protocol that was identical to the experimental protocol except for exposure to intermittent hypoxia (Yokhana et al., 2012). The protocol was repeated each day over a 10-day period (Yokhana et al., 2012). On the initial and final day of the protocol, sleep studies were completed 30 min after the participants were exposed to either the experimental or sham protocol during wakefulness (Yokhana et al., 2012). Despite the expression of ventilatory long-term facilitation during wakefulness, the number and duration of apneas/hypopneas increased during sleep indicating that ventilatory and upper airway muscle long-term facilitation did not manifest during this state (Yokhana et al., 2012). The arousal state (see Wake vs. Sleep for further discussion) and uncontrolled levels of carbon dioxide that resulted in hypocapnia during sleep likely limited the expression of ventilatory and upper airway long-term facilitation. Though unexpected, a reduction in the number of breathing events after 10 days of sham (hypercapnia alone) exposure was reported in the control group (Yokhana et al., 2012). The exposure to sustained levels of carbon dioxide might have resulted in a widening of the carbon dioxide reserve, ultimately promoting breathing stability (Dempsey et al., 2010). However, further experiments exploring this plausibility are required.
The increase in apnea frequency and duration was similar to the results obtained in a subsequent study, which demonstrated an increase in apnea-hypopnea index following acute exposure to intermittent hypoxia (i.e. twelve 2-min hypoxic episodes with sustained hypercapnia - PETCO2 sustained at 3 Torr above baseline) compared to the sham protocol (hypercapnia only) during sleep in both men and women with obstructive sleep apnea (Syed et al., 2013). To explore the mechanism responsible for the increased apnea frequency and duration following intermittent hypoxia exposure, loop gain and the arousal threshold during non-rapid eye movement sleep immediately following exposure to intermittent hypoxia during wakefulness were measured (Alex et al., 2019). The results showed that exposure to mild forms of intermittent hypoxia increases loop gain and the arousal threshold (Alex et al., 2019). These modifications could lead to increases in apnea frequency and duration in individuals with obstructive sleep apnea (Alex et al., 2019). Thus, exposure to intermittent hypoxia during sleep might result in an increase in apnea severity and duration. This outcome is likely in response to an increased ventilatory sensitivity to hypoxia/hypercapnia that leads to hypocapnia secondary to arousal induced hyperventilation (Mateika et al., 2015; Mateika and Narwani, 2009; Mateika and Syed, 2013). The hypocapnia coupled to a reduced carbon dioxide reserve likely leads to the promotion of apneic events (Mateika et al., 2015; Mateika and Narwani, 2009; Mateika and Syed, 2013).
8. Autonomic, cardiovascular & hemodynamic responses to intermittent hypoxia
In addition to studies that have explored respiratory plasticity in humans, numerous studies have also explored the impact of hypoxia on autonomic plasticity and the accompanying cardiovascular (Bernardi et al., 2001; Chacaroun et al., 2016; Cutler et al., 2004a; Cutler et al., 2004b; Gilmartin et al., 2010; Jacob et al., 2020; Jouett et al., 2017; Moradkhan et al., 2010; Ott et al., 2020; Stuckless et al., 2020; Xie et al., 2001) and hemodynamic outcomes (Cutler et al., 2004b; Gilmartin et al., 2010; Moradkhan et al., 2010; Stuckless et al., 2020; Tamisier et al., 2011; Xie et al., 2001). The protocols used to explore the impact of intermittent hypoxia on autonomic plasticity and its associated outcomes in humans are even more variable than those used to explore respiratory plasticity (See Excel Spreadsheet). In some cases, intermittent hypoxia was employed as the primary stimulus (Bernardi et al., 2001; Chacaroun et al., 2016; Cutler et al., 2004a; Cutler et al., 2004b; Gilmartin et al., 2010; Tamisier et al., 2011). However, the induction of hypoxia coupled to intermittent apneic events has also been used (Cutler et al., 2004a; Cutler et al., 2004b; Jouett et al., 2017). Moreover, repeated exposure to intermittent hypoxia protocols has typically taken two forms. Repeated daily exposure to a few, or many, brief episodes of hypoxia or repeated daily exposure to sustained hypoxia for periods that extended beyond 60 min (See Excel Spreadsheet). With this caveat in mind, we have attempted to highlight similar findings across these protocols to explore the beneficial or detrimental autonomic/cardiovascular outcomes linked to exposure to intermittent hypoxia. Ultimately, we have integrated (see Section Integrated summary of responses to mild intermittent hypoxia below) the findings from respiratory and autonomic plasticity studies to provide a unified overview of expected respiratory/autonomic outcomes linked to mild forms of intermittent hypoxia.
9. Augmentation of autonomic & cardiovascular sensitivity during acute exposure to hypoxia
Progressive augmentation of the hypoxic ventilatory response has been defined and discussed above. Similar terminology has not been used in defining modifications in autonomic and cardiovascular responses to hypoxia. However, the premise is analogous to that outlined for the hypoxic ventilatory response. Specifically, studies have explored if sympathetic nerve activity (Cutler et al., 2004b; Morgan et al., 1995), blood pressure or heart rate sensitivity to hypoxia progressively increases from the initial to the final episode of an intermittent hypoxia protocol (Chacaroun et al., 2016; Foster et al., 2009; Foster et al., 2010; Foster et al., 2005; Wadhwa et al., 2008; Wang et al., 2020; Zhang et al., 2014; Zhang et al., 2010). The studies that have adequately quantified this response are few. On the other hand, additional studies have explored the response to a step wise decrease in hypoxia before and following exposure to intermittent hypoxia (Cutler et al., 2004b; Morgan et al., 1995). In all but two studies (Foster et al., 2010; Zhang et al., 2010), these measures were made before and after exposure to intermittent hypoxia over several consecutive days.
A progressive increase in heart rate from the initial to the final episode during a one-time exposure to intermittent hypoxia while carbon dioxide was sustained slightly above baseline has been reported (Wadhwa et al., 2008). This response was coupled to a progressive increase in sympathovagal balance and a progressive reduction in parasympathetic nervous system activity (as determined from measures of heart rate variability) in healthy males. Although evident in men, these modifications were not evident or were less clear in women (see Sex below for further discussion) (Wadhwa et al., 2008). Zhang et al. (2010 &2014) also reported evidence of progressive augmentation of heart rate in a group of healthy men and women exposed to intermittent hypoxia [see Fig. 4 in (Zhang et al., 2010) and Fig. 5 in (Zhang et al., 2014)]. The tachycardic response to reductions in the partial pressure of end-tidal oxygen progressively increased from the 1st to 5th episode. Caution is required in interpreting the data since heart rate during the 5th episode did not exceed values measured during the 1st episode until the lowest values of the partial pressure of end-tidal oxygen were achieved. Consequently, a lower baseline value prior to the 5th episode was responsible for the result. In addition to these findings, Foster and colleagues [see (Foster et al., 2009; Foster et al., 2010; Foster et al., 2005)] in a series of investigations showed that the blood pressure response to hypoxia is increased during the last compared to the initial episode of hypoxia (Foster et al., 2005), or during the last half compared to the initial half of an intermittent protocol (Foster et al., 2009; Foster et al., 2010) (Fig. 7). Thus, there is evidence to support the notion that heart rate and blood pressure sensitivity to a given level of hypoxia is enhanced from the beginning to the end of intermittent hypoxia protocol.
Fig. 7.
Example of a 6 h exposure to intermittent hypoxia in a single subject. Left panel displays the first 30 min of intermittent hypoxia. The middle panel shows the following 5 h of exposure and the right panel displays the final 30 min of exposure. Note that the blood pressure response to hypoxia was greater at the end compared to the beginning of the protocol, despite a similar level of hypoxia. The traces from top to bottom are: end-tidal partial pressure of oxygen (PETO2), arterial oxyhemoglobin saturation (SaO2), end-tidal partial pressure of carbon dioxide (PETCO2), mean arterial pressure (MAP), and middle cerebral artery peak blood flow velocity (VP). The red lines indicate the upper and lower bounds of mean arterial pressure during baseline and then after 6 h of intermittent hypoxia. The blue lines with red arrows indicate the upward shift in mean arterial pressure that is occurring during the 6 h of intermittent hypoxia. The red lines for oxygen saturation show similar degrees of oxygen saturation throughout the study. Also note, in this figure cerebral blood flow velocity did not change. Reprinted with permission from “Cardiovascular and cerebrovascular responses to acute hypoxia following exposure to intermittent hypoxia in healthy humans” by G.E. Foster et al. J. Physiol. 587:3287–3299, 2009.
In addition, a couple of studies (Cutler et al., 2004b;Morgan et al., 1995) have shown that muscle sympathetic nerve activity in response to step-wise decreases in oxygen saturation is enhanced after a one-time exposure to intermittent hypoxic apneas (Cutler et al., 2004b) or after a one-time exposure to 20 min of sustained isocapnic hypoxia (Morgan et al., 1995) (NB the enhanced response after sustained isocapnic hypoxia did not achieve statistical significance P = 0.07). Interestingly, in contrast to the findings in the previous paragraph, the corresponding blood pressure responses were not different from baseline [see Fig. 4 in (Cutler et al., 2004a)] (See Mechanistic underpinnings of autonomic and cardiovascular plasticity during and following exposure to hypoxia for further discussion below).
10. Autonomic & cardiovascular responses following acute exposure to intermittent hypoxia
Assessing autonomic and cardiovascular plasticity during recovery from acute intermittent hypoxia is difficult because of the absence of a standard protocol. Nonetheless, results from publications which utilized brief episodes (i.e. less than 5 min) of hypoxia (Ainslie et al., 2007; Chacaroun et al., 2016; Cutler et al., 2004a; Cutler et al., 2004b; Jouett et al., 2017) or hypoxia/hypercapnia (Cutler et al., 2004a; Cutler et al., 2004b; Jacob et al., 2020; Ott et al., 2020; Somers et al., 1991; Stuckless et al., 2020; Vermeulen et al., 2020) with (Cutler et al., 2004a; Cutler et al., 2004b; Jouett et al., 2017) or without accompanying short apneas for less than 2 h indicate that following an acute exposure to intermittent hypoxia sustained increases in muscle sympathetic nerve activity (Cutler et al., 2004a; Cutler et al., 2004b; Ott et al., 2020), or modifications in the firing pattern of the nerve (Ott et al., 2020), are typically evident in healthy males and females. Likewise, modifications in heart rate variability indicative of increases in sympathetic activity and decreases in parasympathetic activity have also been reported (Chacaroun et al., 2016; Wadhwa et al., 2008), although not in all cases (Chacaroun et al., 2016). Increases in muscle sympathetic nervous system activity are generally accompanied by corresponding increases in diastolic and/or mean arterial pressure (Jouett et al., 2017; Ott et al., 2020). Although less frequently, the absence of a change in blood pressure has also been reported (Cutler et al., 2004a; Cutler et al., 2004b). The lack of consistency regarding blood pressure measures may in part be linked to sex differences (Jacob et al., 2020) (see Factors that impact the magnitude of autonomic and cardiovascular plasticity - Sex). On the other hand, variations in the types of intermittent hypoxia protocols employed might also account for the lack of consistency (see Excel spreadsheet). These protocols might activate different mechanisms that compete with increases in sympathetic activity to varying degrees ultimately resulting in different blood pressure responses (see Mechanistic Underpinning of Autonomic and Cardiovascular Plasticity for further details).
In addition to modifications in blood pressure, Stuckless et al. (2020) reported that exposure to a few brief episodes of hypoxia or hypoxia hypercapnia is also accompanied by reductions in brachial blood flow, forearm vascular conductance and mean sheer rate (Stuckless et al., 2020). These modifications are also accompanied by increases in systemic, but not regional forearm, neurovascular transduction (Stuckless et al., 2020). Likewise, exposure to intermittent hypoxia hypercapnia reportedly leads to increases in posterior and middle cerebral artery neurovascular transduction (Vermeulen et al., 2020).
An acute one-time exposure to 20, 30 and 60 min of sustained hypoxia at 80% SaO2 results in sustained increases in muscle sympathetic nervous system activity for up to twenty minutes following the hypoxic exposure (Lusina et al., 2006; Morgan et al., 1995; Querido et al., 2010; Tamisier et al., 2005; Xie et al., 2001) (Fig. 8). Despite the sustained increase in muscle sympathetic nervous system activity, no accompanying increase in heart rate or blood pressure has typically been reported (Beaudin et al., 2015; Chacaroun et al., 2016; Lusina et al., 2006; Tamisier et al., 2005; Xie et al., 2001), although there are exceptions (Querido et al., 2010). Thus, short term exposure to sustained hypoxia appears to elicit a sustained increase in muscle sympathetic nervous system activity, which is similar to intermittent hypoxia. This similarity in autonomic plasticity contrasts with responses linked to respiratory plasticity because sustained increases in ventilation (i.e. long-term facilitation) are observed following exposure to intermittent hypoxia but not after exposure to sustained hypoxia (McEvoy et al., 1996; Querido et al., 2010; Tamisier et al., 2005; Xie et al., 2001). Indeed, following acute exposure to sustained hypoxia ventilation usually returns immediately to baseline despite sustained increases in muscle sympathetic nervous system activity (see Mechanistic underpinnings of autonomic and cardiovascular plasticity during acute exposure to intermittent hypoxia for further details) (Lusina et al., 2006; Morgan et al., 1995; Tamisier et al., 2005; Xie et al., 2001). It is also interesting to note that in contrast to intermittent hypoxia, a one-time exposure to sustained hypoxia often leads to increases in muscle sympathetic nervous system activity with no accompanying modifications in blood pressure, leg blood flow or vascular resistance (Morgan et al., 1995; Xie et al., 2001), tissue oxygenation or muscle and prefrontal oxy-hemoglobin concentration (Chacaroun et al., 2016). There is one exception to this generalization, since Tamisier and colleagues (Tamisier et al., 2004) reported that exposure to acute sustained hypoxia led to increases in muscle sympathetic nervous system activity and mean arterial pressure, which was not evident after exposure to intermittent hypoxia. The reason for this discrepancy is not entirely clear, but it should be noted that the intensity of the hypoxic exposure was significantly lower during sustained (SaO2 = 85%) vs. intermittent hypoxia (SaO2 = 92%). Collectively, published findings indicate that increases in muscle sympathetic activity are evident following a one-time exposure to a few brief episodes of intermittent hypoxia or a one-time exposure to sustained hypoxia. Increases in blood pressure often accompany the increase in muscle sympathetic activity following exposure to intermittent hypoxia. In contrast, blood pressure responses are less likely to be elevated following a one-time exposure to sustained hypoxia.
Fig. 8.
Effect of isocapnic hypoxia and hyperoxia on muscle sympathetic nerve activity (MSNA). Representative traces represent 30 s of data obtained from one subject at baseline, during exposure to isocapnic hypoxia, during recovery (4.5 min after end of hypoxia), and during exposure to hyperoxia (5.5 min after end of hypoxia). Note that blood pressure during recovery following exposure to hypoxia was similar to baseline. In contrast, muscle sympathetic nerve activity was increased following exposure to hypoxia. Also note that the administration of hyperoxia decreases the burst frequency of muscle sympathetic activity during recovery. The red line highlights the upper (systolic) and lower (diastolic) boundaries of blood pressure. BP, blood pressure; PETCO2, end-tidal partial pressure of carbon dioxide. VT, tidal volume; MAP, mean arterial blood pressure; SpO2, oxyhemoglobin saturation. Reprinted with permission from “Hyperoxia attenuates muscle sympathetic nerve activity following isocapnic hypoxia in humans” by J.S. Querido et al., J. Appl. Physiol. 108: 906–912, 2010.
11. Factors that impact the magnitude of autonomic and cardiovascular plasticity
11.1. Sex
A progressive increase in sympathovagal balance and heart rate, coupled to a progressive reduction in parasympathetic nervous system activity, is evident in men but not women during exposure to intermittent hypoxia. Instead, reductions in parasympathetic nervous system activity and increases in heart rate occur immediately (i.e. the initial hypoxic episode) and remain unchanged throughout the remainder of the protocol in women (Wadhwa et al., 2008). Although, a progressive increase in sympathetic nervous system activation is evident in both men and women, a greater increase is ultimately achieved in men. These sex differences might indicate that autonomic responses (i.e. vagal withdraw and increases in sympathetic nervous system activity) are faster in women, as previously speculated based on autonomic responses to a single bout of hypoxia (Jones et al., 1999). Despite findings of sex differences in autonomic nervous system activity and heart rate, a progressive increase in blood pressure in response to brief episodes of hypoxia has only been reported in males at the present time (Foster et al., 2009; Foster et al., 2010; Foster et al., 2005; Shatilo et al., 2008; Wang et al., 2020).
Sex differences may also be evident following exposure to hypoxia (See Excel Sheet). Sustained increases in heart rate and sympathovagal balance coupled to decreases in parasympathetic nervous system activity were evident in men but not women throughout a 15-min recovery period following acute intermittent hypoxia (Wadhwa et al., 2008). In women, heart rate remained elevated during the first 3-min following hypoxia but returned to baseline, providing further support for the hypothesis that the autonomic response to a given perturbation may be faster in women compared to men (Jones et al., 1999). On the other hand, this speculation is not supported by the recent findings of Jacob and colleagues which showed that adjustments in muscle sympathetic nerve activity following exposure to intermittent hypoxia was similar in males compared to females (Jacob et al., 2020). Interestingly, despite this similarity, Jacob and colleagues also reported that blood pressure was sustained above baseline after exposure to intermittent hypoxia in males but not females (Jacob et al., 2020). Despite the outlined comparisons between sexes, this assessment is typically not completed, despite the inclusion of men and women in many studies (Cutler et al., 2004a; Cutler et al., 2004b; Jones et al., 1999; Moradkhan et al., 2010; Ott et al., 2020; Somers et al., 1991; Tamisier et al., 2005). We assume that this lack of comparison is either because differences between the sexes were not evident or because the sample sizes were too small to reach a meaningful conclusion.
11.2. Carbon dioxide
The impact that carbon dioxide levels have on the sensitivity of autonomic, cardiovascular and hemodynamic responses during or following exposure to intermittent or sustained hypoxia is largely unknown. The reason is that few studies have systematically compared if measures obtained in the presence of isocapnia (Lusina et al., 2006) or slightly elevated levels of carbon dioxide (Cutler et al., 2004a; Cutler et al., 2004b; Morgan et al., 1995; Xie et al., 2001) are similar to measures obtained under conditions of hypocapnia (Ainslie et al., 2007; Foster et al., 2009). However, indirect evidence indicates that the maintenance of carbon dioxide may not be important when measuring the sensitivity of blood pressure to hypoxia. An increase in the blood pressure response is evident independent of whether carbon dioxide levels are maintained (i.e. isocapnic) (Cutler et al., 2004b; Foster et al., 2005; Lusina et al., 2006) or uncontrolled (Ainslie et al., 2007; Foster et al., 2009) (i.e. poikilocapnic). Likewise, modifications in autonomic, cardiovascular or hemodynamic measures are similar when carbon dioxide levels are reduced or maintained during recovery, compared to baseline (Foster et al., 2009; Morgan et al., 1995; Ott et al., 2020). Alternatively, one outcome measure that might be impacted by carbon dioxide is cerebral blood flow. Modifications in cerebral hemodynamics indicate that the cerebral vasodilatory response to hypoxia might decrease following exposure to daily repeated intermittent hypoxia. However, Ainslie and colleagues provided an alternative explanation by showing that reductions in carbon dioxide, that were correlated to the hypoxic ventilatory response, were also correlated to reductions in cerebral blood flow velocity (Ainslie et al., 2007). Thus, in some cases modifications in carbon dioxide could be responsible for the reductions in cerebral blood flow.
Modifying levels of carbon dioxide have also been used to confirm that enhancement of autonomic and cardiovascular/hemodynamic responses to hypoxia during or following intermittent hypoxia are the consequence of exposure to hypoxia and no other stimulus. Enhancement of blood pressure responses to hypoxia were independent of the level of carbon dioxide during exposure to intermittent hypoxia, which ranged from isocapnia to hypercapnia (Cutler et al., 2004b). Moreover, enhancement of blood pressure responses to normoxic hypercapnic were not evident following exposure to intermittent hypoxia (Foster et al., 2009). Thus, the importance of hypoxia as a stimulus to initiate plasticity, as well as identifying the potential chemoreceptor that mediates the response, was confirmed by exposure to varying levels of carbon dioxide.
11.3. Repeated daily exposure
Several studies have explored if repeated daily exposure to brief episodes of intermittent hypoxia ultimately leads to modifications in heart rate and blood pressure sensitivity to hypoxia. Studies (Ainslie et al., 2007; Foster et al., 2009) have shown that the change in systolic, diastolic and mean arterial blood pressure in response to acute isocapnic or poikilocapnic stepwise reductions in oxygen saturation is enhanced following exposure to intermittent hypoxia for 4 (Foster et al., 2009) or 12 days (Ainslie et al., 2007). In addition, increases in cerebral vascular resistance (Foster et al., 2009), coupled to reductions in cerebral oxygen saturation (Foster et al., 2005) and cerebral blood flow velocity (Ainslie et al., 2007) during isocapnic hypoxia indicate that the cerebral vasodilatory response to hypoxia might decrease following exposure to daily repeated intermittent hypoxia. In contrast, Foster and colleagues reported an increase in cerebral blood flow velocity during the final day (i.e. 4th day) of exposure to intermittent hypoxia (Foster et al., 2009). However, these authors suggested that this response coupled to increases in cerebral vascular resistance indicated the loss of autoregulation.
Repeated daily exposure (i.e. 10 days) to 60 min of sustained hypoxia is accompanied by an enhanced muscle sympathetic nerve response to hypoxia (Lusina et al., 2006). Likewise, repeated daily exposure (i.e. 7 days) to sustained hypoxia (i.e. 1 h each day) (Katayama et al., 2001) or exposure to sustained hypoxia for a period of 12 days (Ainslie et al., 2007) also enhances the blood pressure response to hypoxia. Indeed, studies specifically designed to compare responses to protocols that employ sustained vs. intermittent hypoxia protocols have reported similar cardiovascular and cerebrovascular outcomes as reported in the previous paragraph (Ainslie et al., 2007; Chacaroun et al., 2016; Foster et al., 2009; Foster et al., 2010; Foster et al., 2005). Nonetheless, it should be noted that in a few cases differences in heart rate variability (Chacaroun et al., 2016) and blood pressure responses [(Foster et al., 2005) Fig. 5] have been reported, but the differences were small and largely the exception. Collectively, most of the published work suggests that the muscle sympathetic nerve and blood pressure response to hypoxia is enhanced and the cerebral vasodilatory response is decreased following repeated daily exposure to brief episodes of hypoxia or sustained hypoxia. It should be noted however, that an increase in blood pressure sensitivity to hypoxia was not evident in sedentary and healthy older men after exposure to intermittent hypoxia each day for 10 days (Shatilo et al., 2008) or after exposure to 60 min of sustained hypoxia each day for 10 days (Lusina et al., 2006). Although the reasons for the differences are not clear, the number of episodes that participants were exposed to each day in Shatilo and colleague study were few (i.e. 4 per day) (Shatilo et al., 2008).
Independent of the stimulus (repeated intermittent or sustained hypoxia), increases in the muscle sympathetic response, or blood pressure response, to hypoxia are often coupled to increases in the hypoxic ventilatory response (Ainslie et al., 2007; Foster et al., 2005; Katayama et al., 2001; Lusina et al., 2006). This finding indicates that a similar mechanism might mediate the cardiovascular and ventilatory responses (see Mechanistic Underpinnings below).
In contrast to autonomic and cardiovascular responses to hypoxia (NB sustained or intermittent), no direct measures of autonomic plasticity have been obtained following repeated daily exposure to a few brief episodes of hypoxia (i.e. 3 to 10) over a short duration of exposure (i.e. less than 2 h) (Bernardi et al., 2001; Chacaroun et al., 2016; Zhang et al., 2014). In a few studies, non-invasive measures of heart rate variability were determined before and following daily exposure that ranged from 3 to 14 days (Bernardi et al., 2001; Chacaroun et al., 2016; Zhang et al., 2014). The results to date are equivocal, with studies indicating that measures of heart rate variability in the frequency domain are similar before and after repeated daily exposure (Bernardi et al., 2001; Chacaroun et al., 2016). On the other hand, heart rate variability measures in the frequency and time domain have also indicated that parasympathetic nervous system activity is enhanced after daily exposure to a few brief episodes of hypoxia (Zhang et al., 2014). In conjunction with these findings, heart rate is reportedly similar (Ainslie et al., 2007; Bernardi et al., 2001; Chacaroun et al., 2016; Foster et al., 2005; Querido et al., 2008; Querido et al., 2015; Shatilo et al., 2008), or reduced (Serebrovska et al., 2017; Zhang et al., 2014; Zhang et al., 2015) compared to baseline. In addition, Ainslie et al. and Zhang et al. reported that cerebral artery velocity under resting conditions was unchanged following daily exposure to intermittent hypoxia over 12 (Ainslie et al., 2007) and 14 days (Zhang et al., 2015).
The most widely studied variable following repeated daily exposure to a few brief episodes of hypoxia is blood pressure. In some cases, systolic, diastolic and mean arterial pressure were reported to be similar before and after exposure (Bernardi et al., 2001; Chacaroun et al., 2016; Foster et al., 2005; Haider et al., 2009; Querido et al., 2008; Zhang et al., 2014; Zhang et al., 2015). However, there is evidence that systolic (Burtscher et al., 2009; Chacaroun et al., 2016; Lyamina et al., 2011; Muangritdech et al., 2020; Mukharliamov et al., 2006; Serebrovska et al., 2017; Shatilo et al., 2008), diastolic (Burtscher et al., 2009; Lyamina et al., 2011; Muangritdech et al., 2020; Wang et al., 2020) and mean arterial pressure (Lyamina et al., 2011; Muangritdech et al., 2020; Wang et al., 2020) are reduced after repeated daily exposure. The reductions in blood pressure have been observed in a variety of populations including healthy controls (Chacaroun et al., 2016) and individuals with chronic obstructive pulmonary disease (Burtscher et al., 2009), hypertension (Burtscher et al., 2004; Korkushko et al., 2010; Lyamina et al., 2011; Mukharliamov et al., 2006; Serebrovska et al., 2017; Shatilo et al., 2008) and myocardial infraction (Burtscher et al., 2004). Collectively, the results obtained suggest that repeated daily exposure to a few brief episodes of hypoxia is not detrimental, and indeed in many cases leads to positive outcomes measures as indicated by the reported significant reductions in blood pressure.
Although positive outcome measures have been reported following repeated daily exposure to a few brief episodes of hypoxia, the opposite occurs when individuals are repeatedly exposed to numerous brief episodes of hypoxia over a long duration of exposure (i.e. 6–9 h in a given day) (Foster et al., 2009; Foster et al., 2010; Gilmartin et al., 2010; Pialoux et al., 2009; Tamisier et al., 2011). In contrast to the heart rate variability results outlined in the previous paragraph, measures of muscle sympathetic nervous system activity increased following repeated daily exposure to numerous brief episodes of hypoxia (Gilmartin et al., 2010; Tamisier et al., 2011). Moreover, the magnitude of the increase in muscle sympathetic nervous system activity was greater than typically reported after a single day exposure to intermittent hypoxia (Gilmartin et al., 2010; Tamisier et al., 2011). The increase in muscle sympathetic nervous system activity was not accompanied by an increase in heart rate, if reported (Foster et al., 2009; Gilmartin et al., 2010; Tamisier et al., 2011). In contrast, increases in systolic (Tamisier et al., 2011), diastolic (Foster et al., 2009; Gilmartin et al., 2010; Tamisier et al., 2011) or mean arterial pressure (Foster et al., 2009; Pialoux et al., 2009; Tamisier et al., 2011) have been reported. In one case, only diastolic pressure increased (Ott et al., 2020). The sustained increases in pressure were also accompanied by other detrimental outcomes including reductions in forearm blood flow (Gilmartin et al., 2010). Likewise, left ventricular end-diastolic diameter significantly decreased while aortic diameter and left ventricular end-systolic diameter tended to increase (P = 0.07) and decrease (P = 0.06) respectively, following 10–14 consecutive days of intermittent poikilocapnic hypoxia administered during wakefulness (Saeed et al., 2012) or sleep (Gilmartin et al., 2010; Tamisier et al., 2011). The increase in muscle sympathetic nervous system activity was strongly correlated to decreases in left ventricular end-systolic and end-diastolic diameter, which was accompanied by unchanged values in ejection fraction, stroke volume and cardiac output (Tamisier et al., 2011). Given the accompanying increase in blood pressure these results indicate that in addition to increases in forearm vascular resistance, increases in systematic vascular resistance also occurred after exposure to brief episodes of hypoxia for more than 6 h (Gilmartin et al., 2010; Tamisier et al., 2011). Overall, the evidence presented in this and the previous paragraph indicate that autonomic and cardiovascular outcomes are distinctly different based on the dose of intermittent hypoxia administered. A long duration of exposure coupled to severe hypoxemia will likely lead to detrimental outcome measures while beneficial outcomes are linked to milder doses (see Mechanistic Underpinning for additional discussion).
A few studies have also explored the effects of exposure to repeated daily sustained hypoxia on autonomic or cardiovascular function. Muscle sympathetic nerve activity after compared to before repeated daily exposure to sustained hypoxia is increased (Lusina et al., 2006). Reductions in sympathovagal balance have also been reported following 10 days of exposure to 60 min of hypoxia in healthy normotensive individuals (Taralov et al., 2018). Moreover, this group and others (Ainslie et al., 2007;Chacaroun et al., 2016; Foster et al., 2005) have reported that resting blood pressure (mean arterial, systolic and diastolic blood pressure) measures remain unaltered after compared to before exposure to repeated daily sustained hypoxia. Besides measures of blood pressure, it has also been reported that mean cerebral arterial blood flow velocity and cerebral tissue oxygen saturation remains unaltered following exposure to repeated daily sustained hypoxia (Querido et al., 2009). Thus, reductions in blood pressure may be clearer after repeated daily exposure to a few brief episodes of intermittent hypoxia vs. sustained hypoxia.
At present the impact of repeated daily exposure to brief episodes of hypoxia for a short duration each day on muscle sympathetic activity is unknown. However, outcome measures indicate that this paradigm typically leads to reductions in blood pressure. In contrast, repeated daily exposure to intermittent hypoxia for a duration of 6 h or greater is typically accompanied by increases in blood pressure. Finally, repeated daily exposure to sustained hypoxia promotes increases in sympathetic activity. However, this increase is typically unaccompanied by modifications in blood pressure.
11.4. Timing of application
We are currently unaware of studies that have explored the effect that time of day has on the impact of repetitive mild intermittent hypoxia on autonomic and cardiovascular function. Indeed, we have suggested that administering repetitive intermittent hypoxia prior to sleep may have negative effects on the severity of sleep apnea (Alex et al., 2019; Panza et al., 2019), but the differential effects on autonomic and cardiovascular function based on the time of administration remains unknown. However, considering the changes in peripheral chemoreceptor sensitivity, baroreceptor sensitivity, and changes in blood pressure reported over a 24-h period, we suspect the time of administration could impact neuroplasticity during hypoxia, as well as, impact the therapeutic potential of repetitive intermittent hypoxia.
11.5. Mechanistic underpinnings of autonomic and cardiovascular plasticity following exposure to hypoxia
11.5.1. Hyperoxia and muscle sympathetic nerve activity
As outlined above, numerous studies have reported that long term sustained increases in muscle sympathetic nerve activity are evident following exposure to intermittent or sustained hypoxia (Gilmartin et al., 2010; Lusina et al., 2006; Tamisier et al., 2004; Tamisier et al., 2011; Xie et al., 2001), but not after exposure to normoxic/hyperoxic hypercapnia (Morgan et al., 1995; Querido et al., 2010). This sustained increase is evident despite differences in the type of protocol that has been administered. These differences include the length of exposure (one - time exposure vs. repeated daily exposure) (Cutler et al., 2004b; Jacob et al., 2020; Ott et al., 2020), the type of exposure (intermittent vs. sustained) (Lusina et al., 2006; Tamisier et al., 2011), the intensity of exposure (Jacob et al., 2020; Moradkhan et al., 2010; Querido et al., 2010) and the inclusion of voluntary apneas during exposure (Cutler et al., 2004a; Cutler et al., 2004b; Jouett et al., 2017).
It has been proposed that an enhanced muscle sympathetic nerve response to hypoxia (Lusina et al., 2006) or persistent increases in baseline muscle sympathetic activity (Tamisier et al., 2005; Tamisier et al., 2004; Tamisier et al., 2011) following exposure to intermittent or sustained hypoxia is a consequence of enhanced peripheral chemoreceptor sensitivity (Prabhakar et al., 2009). This enhanced sensitivity could be synonymous with sensory (i.e. carotid sinus nerve) long-term facilitation. In support of this postulation, the hypoxic ventilatory response, a non-invasive indicator of peripheral chemoreflex sensitivity, is increased following exposure to intermittent hypoxia (Foster et al., 2005; Lusina et al., 2006; Pialoux et al., 2009). Likewise, the ventilatory and muscle sympathetic nerve response to hypoxia are correlated (Lusina et al., 2006), while the transient application of hyperoxia (Morgan et al., 1995; Ott et al., 2020; Querido et al., 2010) attenuates both peripheral chemoreflex activity and muscle sympathetic burst frequency (Fig. 8). Moreover, enhanced muscle sympathetic nerve activity persists following exposure to hypoxia even though baroreflex sensitivity remains unchanged (Jacob et al., 2020). Lastly, enhanced peripheral chemoreflex sensitivity and increased levels of baseline muscle sympathetic activity is often evident in individuals that are exposed nightly to intermittent hypoxia because of sleep apnea (Dempsey et al., 2010; Lavie, 2015), and administration of hyperoxia attenuates muscle sympathetic activity in these patients (Leuenberger et al., 1995).
Despite these findings, some evidence disputes the role of the sustained peripheral chemoreflex activity as the primary mechanism responsible for enhanced sympathetic outflow. Sustained increases in ventilation and muscle sympathetic nerve activity are typically evident after exposure to intermittent hypoxia (see below for further discussion). However, this is often not the case after exposure to sustained hypoxia. Instead, ventilation immediately returns to baseline levels following exposure while enhanced baseline sympathetic nervous system activity persists. This finding is unanticipated given that initiation of peripheral chemoreflex plasticity might be expected to result in sustained increases in both ventilation and muscle sympathetic activity. Morgan and colleagues (Morgan et al., 1995) provided possible alternative mechanisms suggesting (i) that peripheral chemoreflex control of sympathetic outflow might have a greater gain than peripheral chemoreflex control of ventilation or (ii) that central effects of hypoxia might have a differential influence on medullary neurons that control sympathetic outflow compared to ventilation. More specifically, central nervous system hypoxia has been shown to cause simultaneous activation of sympathetic neurons and depression of phrenic nerve output. Lastly, Morgan and colleagues proposed that a facilitatory memory within the central nervous system activated by central or peripheral chemoreceptors might be responsible for sustained increases in baseline sympathoexcitation (Morgan et al., 1995). Although this latter explanation is plausible, it should be noted that in most studies’ exposure to hyperoxia, which attenuated muscle sympathetic nerve activity, was short and likely only inhibited peripheral chemoreflex activity. In addition, if transient hyperoxia had a direct effect on central mechanisms, then reductions in baseline muscle sympathetic nerve activity might be expected. Several investigations have shown that this is not the case (Morgan et al., 1995; Ott et al., 2019; Querido et al., 2010).
In totality, the published evidence suggests that the enhanced muscle sympathetic response to hypoxia or sustained increases in baseline muscle sympathetic nerve activity is initiated by peripheral chemoreflex plasticity. If one accepts this premise, the remaining question is why exposure to intermittent hypoxia tends to result in sustained increases in ventilation and sympathetic activity while sustained hypoxia leads to an uncoupling of the responses.
11.5.2. Angiotensin II, reactive oxygen species and blood pressure
The mechanism responsible for the sustained increase in muscle sympathetic nerve activity is a robust finding that is largely independent of the type of hypoxic protocol employed. On the other hand, the mechanisms responsible for mediating accompanying cardiovascular and hemodynamic outcomes may be influenced by the duration and type of protocol employed. In studies that have reported increases in blood pressure, following a one-time exposure to brief apneic events preceded by 3 breaths of nitrogen, intermittent hypoxia hypercapnia or a longer one-time exposure to intermittent hypoxia (Cutler et al., 2004a; Cutler et al., 2004b; Jouett et al., 2017), elevations in sympathetic nerve activity initiated by sensory long-term facilitation in humans are likely responsible. Initiation of this form of facilitation may be mediated in part by angiotensin II binding to angiotensin II type Ia receptors and the accumulation of reactive oxygen species. Indeed, Jouett and colleagues showed that administration of Losartan, an angiotensin II receptor antagonist, was accompanied by reductions in both muscle sympathetic nerve activity and blood pressure (i.e. systolic and mean arterial pressure) in humans (Jouett et al., 2017).
The mechanisms responsible for the elevation of blood pressure after a short one-time exposure to intermittent hypoxia might be different from those responsible for modifying blood pressure after one-time exposures of longer duration (Foster et al., 2009; Foster et al., 2010) or repeated daily exposure to hypoxia (Foster et al., 2009). However, Foster and colleagues proposed that sensory long-term facilitation initiated by angiotensin II production is the starting point of a mechanistic continuum. Indeed, Foster and colleagues (Foster et al., 2010) showed that administration of Losartan abolished sustained increases in diastolic and mean arterial pressure induced by a one – time exposure to intermittent hypoxia that occurred over a longer time period (i.e. 6 h) than the period of exposure in Jouett et al. study (i.e. 20 min) (Jouett et al., 2017). Upregulation of angiotensin II via the renin-angiotensin system may also be responsible for increases in reactive oxygen species and reduced nitrous oxide availability, which are evident after 6 h of exposure to intermittent hypoxia (Foster et al., 2009). This premise is further supported by the finding that the administration of Losartan in humans, 6 h prior to exposure to intermittent hypoxia, mitigates increases in oxidative stress and peroxynitrite activity along with decreases in nitric oxide metabolism colleagues (Foster et al., 2010; Pialoux et al., 2011). The link between angiotensin II and oxidative stress is important since the accumulation of reactive oxygen species contributes to the initiation of sensory long-term facilitation in animals (Peng et al., 2003).
11.5.3. The uncoupling of muscle sympathetic nerve activity and cardiovascular/hemodynamic responses
Despite the evidence linking sustained increases in muscle sympathetic nerve activity with increased blood pressure other studies have reported that elevations in muscle sympathetic activity were not accompanied by changes in blood pressure (Cutler et al., 2004a; Cutler et al., 2004b; Morgan et al., 1995; Somers et al., 1991; Tamisier et al., 2004; Xie et al., 2001) or hemodynamic (Ainslie et al., 2007; Cutler et al., 2004b; Morgan et al., 1995; Xie et al., 2001) responses following a one-time exposure to intermittent apneas, intermittent or sustained hypoxia. Several possible explanations have been provided to explain the uncoupling between muscle sympathetic activity and blood pressure or hemodynamic responses. These include (i) that stimulation of the peripheral chemoreceptors have opposite effects on vascular resistance in skin and muscle. Thus, vasoconstriction in the muscle might be offset by vasodilation in skin or (ii) local vasodilatory effects of systemic hypoxia overrides the vasoconstrictor influence of increased sympathetic outflow so that vascular resistance remains unchanged. Independent of the mechanism, the unchanged vascular resistance might explain the accompanying unaltered measures of blood pressure that have been reported following exposure to hypoxia. Despite these explanations it is somewhat perplexing as to why blood pressure does not increase during recovery from a one-time exposure to sustained hypoxia since sympathetic activity is sustained and the local vascular effects of hypoxia should have dissipated. Xie and colleagues speculated that either the amount of sympathetic activation was insufficient to raise total peripheral resistance or the exposure was not long enough to engage pro-hypertensive mechanisms such as the renin-angiotensin system (Xie et al., 2001).
The potential uncoupling of muscle sympathetic nerve activity and blood pressure is also prevalent following exposure to daily repeated exposure to intermittent hypoxia. As mentioned above, repeated daily exposure to intermittent hypoxia leads to increased sensitivity of the hypoxia ventilatory response and long-term facilitation along with enhance muscle sympathetic nerve activity (Lusina et al., 2006) in humans. In line with the published findings on ventilatory plasticity, enhanced increases in muscle sympathetic nerve activity would presumably lead to increases in blood pressure. However, this is often not the case in healthy humans where repeated daily exposure to intermittent hypoxia is often accompanied by blood pressure that is unaltered or less often reduced. Moreover, exposure to mild forms of intermittent hypoxia are often associated with reductions in blood pressure in individuals that are hypertensive (Burtscher et al., 2009; Burtscher et al., 2004; Lyamina et al., 2011; Muangritdech et al., 2020; Serebrovska et al., 2017; Shatilo et al., 2008; Wang et al., 2020). The prevailing thought is that intermittent hypoxia may stimulate oxygen sensitive transcription factors that lead to the upregulation of genes, hormones and antioxidant enzymes that ultimately promote reductions in inflammation, angiogenesis and improved endothelial function. Indeed, repeated daily exposure to hypoxia in humans has been accompanied by significant increases in hemoglobin (Bernardi et al., 2001; Burtscher et al., 2004; Tan et al., 2018; Tobin et al., 2020), hypoxia inducible factor 1 alpha (HIF1α) (Gangwar et al., 2019; Muangritdech et al., 2020; Tan et al., 2018), vascular endothelial growth factor (Tan et al., 2018), erythropoietin (Tan et al., 2018) and nitric oxide (Lyamina et al., 2011).
12. Repetitive intermittent hypoxia & beneficial cardiovascular outcomes in patient populations
The association of obstructive sleep apnea with increases in muscle sympathetic nerve activity, hypertension, chronic inflammation and overall cardiovascular risk is well documented (Dempsey et al., 2010; Lavie, 2015). These detrimental outcomes might occur because of intrathoracic pressure swings, frequent arousal from sleep and severe intermittent hypoxia with modulating levels of carbon dioxide ranging from hypocapnia to hypercapnia. Conversely, there is some evidence supporting beneficial outcomes following natural exposure to intermittent hypoxia. Steiner et al. (2010) and Ben Ahmed et al. (2015) stratified patients with obstructive sleep apnea based on their apnea-hypopnea index, and reported an increased number of coronary collateral vessels in patients with 10 or more events per hour compared to patients without obstructive sleep apnea (Ben Ahmed et al., 2015; Steiner et al., 2010). Increased myocardial coronary collateralization of the heart in response to ischemia or hypoxia has also been demonstrated in mice (Faber et al., 2021) and rats (Banai et al., 1994). Increased skeletal muscle capillary proliferation and up-regulation of vascular endothelial growth factor has also been reported in patients with obstructive sleep apnea (Wahlin-Larsson et al., 2009). However, the upregulation of hypoxia inducible factor 1α and vascular endothelial growth factor may be dependent on the degree of hypoxemia in patients with sleep apnea.
In addition to naturally occurring intermittent hypoxia, we also showed that repeated daily exposure to experimentally induced mild intermittent hypoxia resulted in significant decreases in blood pressure in individuals with obstructive sleep apnea (Panza et al., 2020a) (see Upper airway long-term facilitation &therapeutic continuous positive airway pressure). This reduction in blood pressure was accompanied by increases in parasympathetic and decreases in sympathetic nervous system activity as determined by heart rate variability. Thus, mild forms of intermittent hypoxia induced naturally or experimentally may initiate beneficial outcomes. In the case of naturally occurring hypoxia, the beneficial outcomes may be most obvious in those with obstructive sleep apnea with a higher arousal threshold (i.e. less disrupted sleep) without accompanying co-morbidities.
Patients with spinal cord injuries are known to have autonomic, cardiovascular, pulmonary and motor dysfunction (Schilero et al., 2009; Wecht et al., 2020; Williams et al., 2019). Therapeutic intermittent hypoxia might lead to improvements in these outcome measures. To date, most studies have focused on the beneficial effects of mild intermittent hypoxia on recovery of limb motor function (Hayes et al., 2014; Navarrete-Opazo et al., 2017a; Navarrete-Opazo et al., 2017b; Trumbower et al., 2017; Trumbower et al., 2012), with fewer investigations focused on the recovery of respiratory function (Jaiswal et al., 2016; Tester et al., 2014). Interestingly, recovery of limb motor function following exposure to mild intermittent hypoxia is enhanced in individuals with moderate obstructive sleep apnea (Vivodtzev et al., 2020), possibly because of nightly priming with naturally occurring intermittent hypoxia. Less is known about the effects of mild intermittent hypoxia on cardiovascular and autonomic function in individuals with spinal cord injury. Most information related to these systems is based on concerns that mild intermittent hypoxia exacerbates autonomic and cardiovascular dysfunction. Thus, participants have been monitored for dizziness, lightheadedness, cyanosis and autonomic dysreflexia. To date, no adverse events following exposure to intermittent hypoxia have been reported. However, objective measures are typically not included. In addition, no change in heart rate (Sankari et al., 2015; Trumbower et al., 2017) and blood pressure (Hayes et al., 2014) was reported following mild intermittent hypoxia. Conversely, a reduction in blood pressure was reported in spinal cord individuals following exposure to intermittent hypoxia combined with ibuprofen supplementation (Lynch et al., 2017). Sankari and colleagues also reported that sympathetic nervous system activity increased during recovery following exposure to intermittent hypoxia in both able-bodied individuals and patients with thoracic injuries, while no change was evident in a group of participants with a cervical spinal cord injury (Sankari et al., 2015). From the currently available evidence, it appears that mild intermittent hypoxia is safe for participants with spinal cord injuries as adverse outcomes have not been reported. Although the few studies completed suggest that beneficial autonomic and cardiovascular outcomes might occur following exposure to mild intermittent hypoxia additional work is required to support this contention.
13. Integrated summary of responses to mild intermittent hypoxia
The use of mild intermittent hypoxia to mitigate apneic events, promote recovery of respiratory and limb motor function in spinal cord injured patients, and reduce blood pressure in patients with hypertension has gained support as a therapeutic tool over the last decade. However, at times the complexity of the physiological response is lost in the excitement of potentially improved outcomes. Knowledge of the integrated respiratory, autonomic and cardiovascular responses allows for the development of protocols that hopefully will serve a beneficial purpose. Based on the information presented in this review, Fig. 9 shows the potential integrated responses to mild intermittent hypoxia and the various factors that impact on the magnitude of the response. Those perturbations (i.e. hyperoxia, losartan) that have been used to explore the mechanisms behind the responses are also included. Based on our review of the literature, a one-time exposure to mild intermittent hypoxia will enhance the ventilatory, autonomic and blood pressure responses to hypoxia (i.e. increase sensitivity of the response), in addition to improving limb motor function. Moreover, following a one-time exposure to mild intermittent hypoxia ventilation and upper airway muscle activity will remain elevated as will sympathetic nervous system activity. More often than not blood pressure will also remain elevated. Thus, both beneficial (long-term facilitation of ventilation and upper airway muscle activity, recovery of limb motor function) and detrimental outcomes (sustained increases in autonomic activity and blood pressure) might be initiated. Thus, doses of intermittent hypoxia that promote the former and minimize the latter is required.
Fig. 9.
A summary of the different forms of respiratory and autonomic plasticity and accompanying outcome measures initiated by exposure to mild forms of intermittent hypoxia. The different forms of plasticity and outcome measures are identified by the blue arrows and boxes. In addition, those factors that influence the magnitude of a given form of plasticity or outcome measure are highlighted in green boxes. Increases in magnitude are indicated by upward pointing red arrows, while decreases in magnitude are indicated by downward pointing red arrows. Sideward pointing red arrows are used to indicate that a given factor has no effect on the magnitude of plasticity.
For example, acute mild intermittent hypoxia activates peripheral chemoreceptors, which in turn initiates progressive augmentation of the hypoxic ventilatory response. The magnitude of this form of plasticity is reduced in the presence of hyperoxia and during sleep compared to wakefulness in humans. In contrast, the magnitude of this form of plasticity is enhanced in the presence of sustained levels of carbon dioxide slightly above baseline, when intermittent hypoxia is administered in the morning compared to the evening and following repeated daily exposure. Increases in progressive augmentation promotes apnea severity in humans. Additionally, oxidative stress has been shown to increase with a single bout of acute intermittent hypoxia (Lee et al., 2009) with concurrent long-term facilitation of minute ventilation that was then mitigated following the administration of antioxidants. Furthermore, evidence from animals shows that when oxidative stress is abolished in the peripheral chemoreceptors, long-term facilitation of ventilation is also abolished, further supporting the role of oxidative stress in long-term facilitation (Peng and Prabhakar, 2003).BP – blood pressure, CSN – carotid sensory nerve, CPAP – continuous positive airway pressure, HR – heart rate, HVR – hypoxic ventilatory response, LTF – Long-term facilitation, MSNA–muscle sympathetic nervous system activity, PA – progressive augmentation, PNSA – parasympathetic nervous system activity.
The outcomes following repeated daily exposure to mild intermittent hypoxia are of great interest. Not only are beneficial forms of respiratory plasticity enhanced in response to repeated exposure, but many studies have shown that dramatic decreases in blood pressure are also induced. Few studies have been completed to determine if these decreases are a consequence of modifications in autonomic nervous system activity. Thus, whether the decline in blood pressure is a consequence of local vasodilatory mechanism in the presence of elevated autonomic responses or is partly or wholly related to beneficial modifications in autonomic nervous system activity remains to be established.
14. Conclusion
The purpose of this review was two-fold. First, we wanted to compile the available evidence on respiratory, autonomic and cardiovascular responses during and following intermittent hypoxia protocols in humans, with a focus on mild intermittent hypoxia. Secondly, we wanted to highlight potential translatable outcomes in humans following mild intermittent hypoxia protocols.
In totality, various hypoxia protocols, continue to show promise as a therapeutic modality, but there are significant gaps in our knowledge of the mechanisms, confounding factors, and most effective therapeutic protocols in humans. In particular, autonomic plasticity and accompanying cardiovascular outcomes following mild intermittent hypoxia requires further study, as does the impact of the time of administration, sex, race, age and carbon dioxide, which are all factors yet to be studied systematically using therapeutic mild intermittent protocols under normobaric conditions.
Supplementary Material
Acknowledgements
This work was supported by awards (I01CX000125, and IK6CX002287 - J.H.M., RX002945 - G.P.) from the Office of Research and Development, Veterans Health Administration, Department of Veterans Affairs and awards (R56 HL-142757, R01 HL-085537 - J.H.M.) from the National Heart, Lung, and Blood Institute.
Footnotes
Declaration of Competing Interest
No conflicts of interest, financial or otherwise, are declared by the author(s).
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.expneurol.2021.113709.
References
- Ainslie PN, Barach A, Cummings KJ, Murrell C, Hamlin M, Hellemans J, 2007.Cardiorespiratory and cerebrovascular responses to acute poikilocapnic hypoxia following intermittent and continuous exposure to hypoxia in humans. J. Appl. Physiol 102, 1953–1961. [DOI] [PubMed] [Google Scholar]
- Alex RM, Panza GS, Hakim H, Badr MS, Edwards BA, Sands SA, Mateika JH,2019. Exposure to mild intermittent hypoxia increases loop gain and the arousal threshold in participants with obstructive sleep apnoea. J. Physiol 597, 3697–3711. [DOI] [PubMed] [Google Scholar]
- Babcock MA, Badr MS, 1998. Long-term facilitation of ventilation in humans duringNREM sleep. Sleep 21, 709–716. [PubMed] [Google Scholar]
- Babcock M, Shkoukani M, Aboubakr SE, Badr MS, 2003. Determinants of long-term facilitation in humans during NREM sleep. J. Appl. Physiol 94, 53–59. [DOI] [PubMed] [Google Scholar]
- Bach KB, Mitchell GS, 1996. Hypoxia-induced long-term facilitation of respiratory activity is serotonin dependent. Respir. Physiol 104, 251–260. [DOI] [PubMed] [Google Scholar]
- Baker TL, Mitchell GS, 2000. Episodic but not continuous hypoxia elicits long-term facilitation of phrenic motor output in rats. J. Physiol 529 (Pt 1), 215–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banai S, Shweiki D, Pinson A, Chandra M, Lazarovici G, Keshet E, 1994. Upregulation of vascular endothelial growth factor expression induced by myocardial ischaemia: implications for coronary angiogenesis. Cardiovasc. Res 28,1176–1179. [DOI] [PubMed] [Google Scholar]
- Bauman WA, La Fountaine MF, Spungen AM, 2014. Age-related prevalence of lowtestosterone in men with spinal cord injury. J. Spinal Cord. Med 37, 32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautista TG, Xing T, Fong AY, Pilowsky PM, 2012. Recurrent laryngeal nerve activity exhibits a 5-HT-mediated long-term facilitation and enhanced response to hypoxia following acute intermittent hypoxia in rat. J. Appl. Physiol 112, 1144–1156. [DOI] [PubMed] [Google Scholar]
- Beaudin AE, Waltz X, Pun M, Wynne-Edwards KE, Ahmed SB, Anderson TJ,Hanly PJ, Poulin MJ, 2015. Human intermittent hypoxia-induced respiratory plasticity is not caused by inflammation. Eur. Respir. J 46, 1072–1083. [DOI] [PubMed] [Google Scholar]
- Behan M, Zabka AG, Mitchell GS, 2002. Age and gender effects on serotonin- dependent plasticity in respiratory motor control. Respir. Physiol. Neurobiol 131,65–77. [DOI] [PubMed] [Google Scholar]
- Ben Ahmed H, Boussaid H, Longo S, Tlili R, Fazaa S, Baccar H, Boujnah MR, 2015. Impact of obstructive sleep apnea in recruitment of coronary collateralityduring inaugural acute myocardial infarction. Ann. Cardiol. Angeiol. (Paris) 64, 273–278. [DOI] [PubMed] [Google Scholar]
- Bernardi L, Passino C, Serebrovskaya Z, Serebrovskaya T, Appenzeller O, 2001. Respiratory and cardiovascular adaptations to progressive hypoxia; effect of intervalhypoxic training. Eur. Heart J 22, 879–886. [DOI] [PubMed] [Google Scholar]
- Berthoud HR, Patterson LM, Sutton GM, Morrison C, Zheng H, 2005. Orexininputs to caudal raphe neurons involved in thermal, cardiovascular, andgastrointestinal regulation. Histochem. Cell Biol 123, 147–156. [DOI] [PubMed] [Google Scholar]
- Bisgard GE, 2000. Carotid body mechanisms in acclimatization to hypoxia. Respir.Physiol 121, 237–246. [DOI] [PubMed] [Google Scholar]
- Bordoni B, Morabito B, Simonelli M, 2020. Ageing of the diaphragm muscle. Cureus12, e6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown EN, Choe Y, Shanahan TL, Czeisler CA, 1997. A mathematical model of diurnal variations in human plasma melatonin levels. Am. J. Phys 272, E506–E516. [DOI] [PubMed] [Google Scholar]
- Brown RE, Basheer R, McKenna JT, Strecker RE, McCarley RW, 2012. Control ofsleep and wakefulness. Physiol. Rev 92, 1087–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burtscher M, Pachinger O, Ehrenbourg I, Mitterbauer G, Faulhaber M, Puhringer R, Tkatchouk E, 2004. Intermittent hypoxia increases exercise tolerancein elderly men with and without coronary artery disease. Int. J. Cardiol 96, 247–254. [DOI] [PubMed] [Google Scholar]
- Burtscher M, Haider T, Domej W, Linser T, Gatterer H, Faulhaber M, Pocecco E,Ehrenburg I, Tkatchuk E, Koch R, Bernardi L, 2009. Intermittent hypoxia increases exercise tolerance in patients at risk for or with mild COPD. Respir. Physiol. Neurobiol 165, 97–103. [DOI] [PubMed] [Google Scholar]
- Cain SW, Chang AM, Vlasac I, Tare A, Anderson C, Czeisler CA, Saxena R, 2017. Circadian rhythms in plasma brain-derived Neurotrophic factor differ in menand women. J. Biol. Rhythm 32, 75–82. [DOI] [PubMed] [Google Scholar]
- Cao Y, Liu C, Ling L, 2010. Glossopharyngeal long-term facilitation requires serotonin 5-HT2 and NMDA receptors in rats. Respir. Physiol. Neurobiol 170,164–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chacaroun S, Borowik A, Morrison SA, Baillieul S, Flore P, Doutreleau S,Verges S, 2016. Physiological responses to two hypoxic conditioning strategies inhealthy subjects. Front. Physiol 7, 675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Tjong YW, Ip SF, Tipoe GL, Fung ML, 2005. Melatonin enhances the hypoxic response of rat carotid body chemoreceptor. J. Pineal Res 38, 157–163. [DOI] [PubMed] [Google Scholar]
- Chowdhuri S, Pierchala L, Aboubakr SE, Shkoukani M, Badr MS, 2008. Long-term facilitation of genioglossus activity is present in normal humans during NREM sleep. Respir. Physiol. Neurobiol 160, 65–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhuri S, Shanidze I, Pierchala L, Belen D, Mateika JH, Badr MS, 2010. Effect of episodic hypoxia on the susceptibility to hypocapnic central apnea duringNREM sleep. J. Appl. Physiol 108, 369–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhuri S, Pranathiageswaran S, Franco-Elizondo R, Jayakar A, Hosni A, Nair A, Badr MS, 2015. Effect of age on long-term facilitation and chemosensitivity during NREM sleep. J. Appl. Physiol 119, 1088–1096. [DOI] [PubMed] [Google Scholar]
- Coria-Lucero CD, Golini RS, Ponce IT, Deyurka N, Anzulovich AC, Delgado SM, Navigatore-Fonzo LS, 2016. Rhythmic Bdnf and TrkB expression patterns in the prefrontal cortex are lost in aged rats. Brain Res. 1653, 51–58. [DOI] [PubMed] [Google Scholar]
- Cutler MJ, Swift NM, Keller DM, Wasmund WL, Burk JR, Smith ML, 2004a. Periods of intermittent hypoxic apnea can alter chemoreflex control of sympathetic nerve activity in humans. Am. J. Physiol. Heart Circ. Physiol 287, H2054–H2060. [DOI] [PubMed] [Google Scholar]
- Cutler MJ, Swift NM, Keller DM, Wasmund WL, Smith ML, 2004b. Hypoxia-mediated prolonged elevation of sympathetic nerve activity after periods of intermittent hypoxic apnea. J. Appl. Physiol 96, 754–761. [DOI] [PubMed] [Google Scholar]
- Dale-Nagle EA, Hoffman MS, Macfarlane PM, Mitchell GS, 2010. Multiplepathways to long-lasting phrenic motor facilitation. Adv. Exp. Med. Biol 669, 225–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Carvalho D, Patrone LG, Taxini CL, Biancardi V, Vicente MC, Gargaglioni LH, 2014. Neurochemical and electrical modulation of the locus coeruleus: contribution to CO2 drive to breathe. Front. Physiol 5, 288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deacon NL, McEvoy DR, Stadler DL, Catcheside PG, 2017. Intermittent hypercapnic hypoxia during sleep does not induce ventilatory long term facilitation in healthy males. J. Appl. Physiol 123, 534–543. [DOI] [PubMed] [Google Scholar]
- Deacon NL, Sands SA, McEvoy DR, Catcheside PG, 2018. Daytime loop gain is elevated in obstructive sleep apnea but not reduced by CPAP treatment. J. Appl. Physiol 125, 1490–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey JA, Veasey SC, Morgan BJ, O’Donnell CP, 2010. Pathophysiology of sleep apnea. Physiol. Rev 90, 47–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis M, Spiegler BJ, Juranek JJ, Bigler ED, Snead OC, Fletcher JM, 2013. Age, plasticity, and homeostasis in childhood brain disorders. Neurosci. Biobehav. Rev 37, 2760–2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devinney MJ, Nichols NL, Mitchell GS, 2016. Sustained hypoxia elicits competing spinal mechanisms of phrenic motor facilitation. J. Neurosci 36, 7877–7885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty BJ, Kopp ES, Watters JJ, 2017. Nongenomic actions of 17-beta estradiol restore respiratory neuroplasticity in young Ovariectomized female rats. J. Neurosci 37, 6648–6660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffin J, 2007. Measuring the ventilatory response to hypoxia. J. Physiol 584, 285–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwinell MR, Janssen PL, Bisgard GE, 1997. Lack of long-term facilitation of ventilation after exposure to hypoxia in goats. Respir. Physiol 108, 1–9. [DOI] [PubMed] [Google Scholar]
- El-Chami M, Shaheen D, Ivers B, Syed Z, Badr MS, Lin HS, Mateika JH, 2014. Time of day affects chemoreflex sensitivity and the carbon dioxide reserve during NREM sleep in participants with sleep apnea. J. Appl. Physiol 117, 1149–1156. [DOI] [PubMed] [Google Scholar]
- El-Chami M, Sudan S, Lin HS, Mateika JH, 2017. Exposure to intermittent hypoxia and sustained hypercapnia reduces therapeutic CPAP in participants with obstructive sleep apnea. J. Appl. Physiol 123, 993–1002. [DOI] [PubMed] [Google Scholar]
- Elliott JE, Greising SM, Mantilla CB, Sieck GC, 2016. Functional impact of sarcopenia in respiratory muscles. Respir. Physiol. Neurobiol 226, 137–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ElMallah MK, Stanley DA, Lee KZ, Turner SM, Streeter KA, Baekey DM,Fuller DD, 2016. Power spectral analysis of hypoglossal nerve activity duringintermittent hypoxia-induced long-term facilitation in mice. J. Neurophysiol 115, 1372–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espana RA, Scammell TE, 2011. Sleep neurobiology from a clinical perspective. Sleep 34, 845–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber JE, Storz JF, Cheviron ZA, Zhang H, 2021. High-altitude rodents haveabundant collaterals that protect against tissue injury after cerebral, coronary andperipheral artery occlusion. J. Cereb. Blood Flow Metab Epub 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogarty MJ, Omar TS, Zhan WZ, Mantilla CB, Sieck GC, 2018. Phrenic motor neuron loss in aged rats. J. Neurophysiol 119, 1852–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster GE, McKenzie DC, Milsom WK, Sheel AW, 2005. Effects of two protocols of intermittent hypoxia on human ventilatory, cardiovascular and cerebral responses to hypoxia. J. Physiol 567, 689–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster GE, Brugniaux JV, Pialoux V, Duggan CT, Hanly PJ, Ahmed SB,Poulin MJ, 2009. Cardiovascular and cerebrovascular responses to acute hypoxia following exposure to intermittent hypoxia in healthy humans. J. Physiol 587, 3287–3299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster GE, Hanly PJ, Ahmed SB, Beaudin AE, Pialoux V, Poulin MJ, 2010. Intermittent hypoxia increases arterial blood pressure in humans through a renin-angiotensin system-dependent mechanism. Hypertension 56, 369–377. [DOI] [PubMed] [Google Scholar]
- Fregosi RF, Mitchell GS, 1994. Long-term facilitation of inspiratory intercostal nerve activity following carotid sinus nerve stimulation in cats. J. Physiol 477 (Pt 3), 469–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangwar A, Pooja Sharma M, Singh K, Patyal A, Bhaumik G, Bhargava K, Sethy NK, 2019. Intermittent normobaric hypoxia facilitates high altitudeacclimatization by curtailing hypoxia-induced inflammation and dyslipidemia.Pflugers Arch. 471, 949–959. [DOI] [PubMed] [Google Scholar]
- Garcia N, Hopkins SR, Powell FL, 2000. Intermittent vs continuous hypoxia: effects on ventilation and erythropoiesis in humans. Wilderness. Environ. Med 11, 172–179. [DOI] [PubMed] [Google Scholar]
- Gerst III DG, Yokhana SS, Carney LM, Lee DS, Badr MS, Qureshi T,Anthouard MN, Mateika JH, 2011. The hypoxic ventilatory response andventilatory long-term facilitation are altered by time of day and repeated daily exposure to intermittent hypoxia. J. Appl. Physiol 110, 15–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmartin GS, Tamisier R, Curley M, Weiss JW, 2008. Ventilatory, hemodynamic, sympathetic nervous system, and vascular reactivity changes after recurrentnocturnal sustained hypoxia in humans. Am. J. Physiol. Heart Circ. Physiol 295, H778–H785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmartin GS, Lynch M, Tamisier R, Weiss JW, 2010. Chronic intermittent hypoxiain humans during 28 nights results in blood pressure elevation and increased musclesympathetic nerve activity. Am. J. Physiol. Heart Circ. Physiol 299, H925–H931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greising SM, Mantilla CB, Gorman BA, Ermilov LG, Sieck GC, 2013. Diaphragmmuscle sarcopenia in aging mice. Exp. Gerontol 48, 881–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greising SM, Ermilov LG, Sieck GC, Mantilla CB, 2015. Ageing and neurotrophicsignalling effects on diaphragm neuromuscular function. J. Physiol 593, 431–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin HS, Pugh K, Kumar P, Balanos GM, 2012. Long-term facilitation of ventilation following acute continuous hypoxia in awake humans during sustainedhypercapnia. J. Physiol 590, 5151–5165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haider T, Casucci G, Linser T, Faulhaber M, Gatterer H, Ott G, Linser A, Ehrenbourg I, Tkatchouk E, Burtscher M, Bernardi L, 2009. Interval hypoxic training improves autonomic cardiovascular and respiratory control in patients with mild chronic obstructive pulmonary disease. J. Hypertens 27, 1648–1654. [DOI] [PubMed] [Google Scholar]
- Harris DP, Balasubramaniam A, Badr MS, Mateika JH, 2006. Long-term facilitation of ventilation and genioglossus muscle activity is evident in the presenceof elevated levels of carbon dioxide in awake humans. Am. J. Phys. Regul. Integr. Comp. Phys 291, R1111–R1119. [DOI] [PubMed] [Google Scholar]
- Hayashi F, Coles SK, Bach KB, Mitchell GS, McCrimmon DR, 1993. Time-dependent phrenic nerve responses to carotid afferent activation: intact vs. decerebellate rats. Am. J. Phys 265, R811–R819. [DOI] [PubMed] [Google Scholar]
- Hayes HB, Jayaraman A, Herrmann M, Mitchell GS, Rymer WZ, Trumbower RD, 2014. Daily intermittent hypoxia enhances walking after chronic spinal cord injury: a randomized trial. Neurology 82, 104–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez C, Abreu J, Abreu P, Castro A, Jimenez A, 2007. Nocturnal melatoninplasma levels in patients with OSAS: the effect of CPAP. Eur. Respir. J 30, 496–500. [DOI] [PubMed] [Google Scholar]
- Hobbins L, Hunter S, Gaoua N, Girard O, 2017. Normobaric hypoxic conditioning tomaximize weight loss and ameliorate cardio-metabolic health in obese populations: asystematic review. Am. J. Phys. Regul. Integr. Comp. Phys 313, R251–R264. [DOI] [PubMed] [Google Scholar]
- Hocker AD, Stokes JA, Powell FL, Huxtable AG, 2017. The impact of inflammation on respiratory plasticity. Exp. Neurol 287, 243–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob DW, Ott EP, Baker SE, Scruggs ZM, Ivie CL, Harper JL, Manrique-Acevedo CM, Limberg JK, 2020. Sex differences in integratedneurocardiovascular control of blood pressure following acute intermittenthypercapnic hypoxia. Am. J. Phys. Regul. Integr. Comp. Phys 319, R626–R636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal PB, Tester NJ, Davenport PW, 2016. Effect of acute intermittent hypoxia treatment on ventilatory load compensation and magnitude estimation of inspiratoryresistive loads in an individual with chronic incomplete cervical spinal cord injury.J. Spinal Cord Med 39, 103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones PP, Davy KP, Seals DR, 1999. Influence of gender on the sympathetic neuraladjustments to alterations in systemic oxygen levels in humans. Clin. Physiol 19, 153–160. [DOI] [PubMed] [Google Scholar]
- Jordan AS, Catcheside PG, O’Donoghue FJ, McEvoy RD, 2002. Long-termfacilitation of ventilation is not present during wakefulness in healthy men or women. J. Appl. Physiol 93, 2129–2136. [DOI] [PubMed] [Google Scholar]
- Jouett NP, Moralez G, Raven PB, Smith ML, 2017. Losartan reduces the immediateand sustained increases in muscle sympathetic nerve activity after hyperacuteintermittent hypoxia. J. Appl. Physiol 122, 884–892. [DOI] [PubMed] [Google Scholar]
- Katayama K, Shima N, Sato Y, Qiu JC, Ishida K, Mori S, Miyamura M, 2001. Effect of intermittent hypoxia on cardiovascular adaptations and response toprogressive hypoxia in humans. High Alt. Med. Biol 2, 501–508. [DOI] [PubMed] [Google Scholar]
- Katayama K, Sato K, Matsuo H, Hotta N, Sun Z, Ishida K, Iwasaki K,Miyamura M, 2005. Changes in ventilatory responses to hypercapnia and hypoxiaafter intermittent hypoxia in humans. Respir. Physiol. Neurobiol 146, 55–65. [DOI] [PubMed] [Google Scholar]
- Kheirandish-Gozal L, Gozal D, 2019. Obstructive sleep apnea and inflammation: proofof concept based on two illustrative cytokines. Int. J. Mol. Sci 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodadadeh B, Badr MS, Mateika JH, 2006. The ventilatory response to carbon dioxide and sustained hypoxia is enhanced after episodic hypoxia in OSA patients. Respir. Physiol. Neurobiol 150, 122–134. [DOI] [PubMed] [Google Scholar]
- Khurram OU, Fogarty MJ, Sarrafian TL, Bhatt A, Mantilla CB, Sieck GC, 2018.Impact of aging on diaphragm muscle function in male and female Fischer 344 rats.Phys. Rep 6, e13786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko ML, King MA, Gordon TL, Crisp T, 1997. The effects of aging on spinalneurochemistry in the rat. Brain Res. Bull 42, 95–98. [DOI] [PubMed] [Google Scholar]
- Koehle MS, Sheel AW, Milsom WK, McKenzie DC, 2007. Two patterns of daily hypoxic exposure and their effects on measures of chemosensitivity in humans.J. Appl. Physiol 103, 1973–1978. [DOI] [PubMed] [Google Scholar]
- Korkushko OV, Shatilo VB, Ishchuk VA, 2010. Effectiveness of intermittent normabaric hypoxic trainings in elderly patients with coronary artery disease. Adv.Gerontol 23, 476–482. [PubMed] [Google Scholar]
- Lavie L, 2015. Oxidative stress in obstructive sleep apnea and intermittent hypoxia–revisited–the bad ugly and good: implications to the heart and brain. Sleep Med. Rev 20, 27–45. [DOI] [PubMed] [Google Scholar]
- Lee DS, Badr MS, Mateika JH, 2009. Progressive augmentation and ventilatorylong-term facilitation are enhanced in sleep apnoea patients and are mitigated by antioxidant administration. J. Physiol 587, 5451–5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuenberger U, Jacob E, Sweer L, Waravdekar N, Zwillich C, Sinoway L, 1995. Surges of muscle sympathetic nerve activity during obstructive apnea are linked to hypoxemia. J. Appl. Physiol 79, 581–588. [DOI] [PubMed] [Google Scholar]
- Lin CC, 1994. Effect of nasal CPAP on ventilatory drive in normocapnic and hypercapnic patients with obstructive sleep apnoea syndrome. Eur. Respir. J 7, 2005–2010. [PubMed] [Google Scholar]
- Ling L, Fuller DD, Bach KB, Kinkead R, Olson EB Jr., Mitchell GS, 2001. Chronic intermittent hypoxia elicits serotonin-dependent plasticity in the centralneural control of breathing. J. Neurosci 21, 5381–5388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HW, Chen YJ, Lai YC, Huang CY, Huang YL, Lin MT, Han SY, Chen CL, Yu CJ, Lee PL, 2017. Combining MAD and CPAP as an effective strategy fortreating patients with severe sleep apnea intolerant to high-pressure PAP and unresponsive to MAD. PLoS One 12, e0187032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Ma Y, Ouyang R, Zeng Z, Zhan Z, Lu H, Cui Y, Dai Z, Luo L, He C,Li H, Zong D, Chen Y, 2020. The relationship between inflammation and neurocognitive dysfunction in obstructive sleep apnea syndrome. J. Neuroinflammation 17, 229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardi G, Mondaini N, Macchiarella A, Del Popolo G, 2007. Female sexual dysfunction and hormonal status in spinal cord injured (SCI) patients. J. Androl 28, 722–726. [DOI] [PubMed] [Google Scholar]
- Lozo T, Komnenov D, Badr MS, Mateika JH, 2017. Sex differences in sleep disordered breathing in adults. Respir. Physiol. Neurobiol 245, 65–75. [DOI] [PubMed] [Google Scholar]
- Lusina SJ, Kennedy PM, Inglis JT, McKenzie DC, Ayas NT, Sheel AW, 2006. Long-term intermittent hypoxia increases sympathetic activity and chemosensitivityduring acute hypoxia in humans. J. Physiol 575, 961–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyamina NP, Lyamina SV, Senchiknin VN, Mallet RT, Downey HF, Manukhina EB, 2011. Normobaric hypoxia conditioning reduces blood pressure and normalizes nitric oxide synthesis in patients with arterial hypertension.J. Hypertens 29, 2265–2272. [DOI] [PubMed] [Google Scholar]
- Lynch M, Duffell L, Sandhu M, Srivatsan S, Deatsch K, Kessler A, Mitchell GS,Jayaraman A, Rymer WZ, 2017. Effect of acute intermittent hypoxia on motorfunction in individuals with chronic spinal cord injury following ibuprofenpretreatment: a pilot study. J. Spinal Cord. Med 40, 295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacFarlane PM, Mitchell GS, 2008. Respiratory long-term facilitation following intermittent hypoxia requires reactive oxygen species formation. Neuroscience 152, 189–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallet RT, Manukhina EB, Ruelas SS, Caffrey JL, Downey HF, 2018.Cardioprotection by intermittent hypoxia conditioning: evidence, mechanisms, and therapeutic potential. Am. J. Physiol. Heart Circ. Physiol 315, H216–H232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateika JH, 2019. A reminder that experimentally induced intermittent hypoxia is an incomplete model of obstructive sleep apnea and its outcome measures. J. Appl. Physiol 127, 1620–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateika JH, Fregosi RF, 1997. Long-term facilitation of upper airway muscleactivities in vagotomized and vagally intact cats. J. Appl. Physiol 82, 419–425. [DOI] [PubMed] [Google Scholar]
- Mateika JH, Komnenov D, 2017. Intermittent hypoxia initiated plasticity in humans: amultipronged therapeutic approach to treat sleep apnea and overlapping co-morbidities. Exp. Neurol 287, 113–129. [DOI] [PubMed] [Google Scholar]
- Mateika JH, Narwani G, 2009. Intermittent hypoxia and respiratory plasticity in humans and other animals: does exposure to intermittent hypoxia promote ormitigate sleep apnoea? Exp. Physiol 94, 279–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateika JH, Sandhu KS, 2011. Experimental protocols and preparations to study respiratory long term facilitation. Respir. Physiol. Neurobiol 176, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateika JH, Syed Z, 2013. Intermittent hypoxia, respiratory plasticity and sleep apnea in humans: present knowledge and future investigations. Respir. Physiol. Neurobiol 188, 289–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateika JH, Mendello C, Obeid D, Badr MS, 2004. Peripheral chemoreflex responsiveness is increased at elevated levels of carbon dioxide after episodic hypoxia in awake humans. J. Appl. Physiol 96, 1197–1205. [DOI] [PubMed] [Google Scholar]
- Mateika JH, El-Chami M, Shaheen D, Ivers B, 2015. Intermittent hypoxia: a low-risk research tool with therapeutic value in humans. J. Appl. Physiol 118, 520–532. [DOI] [PubMed] [Google Scholar]
- Mateika JH, Panza G, Alex R, El-Chami M, 2018. The impact of intermittent or sustained carbon dioxide on intermittent hypoxia initiated respiratory plasticity. What is the effect of these combined stimuli on apnea severity? Respir. Physiol.Neurobiol 256, 58–66. [DOI] [PubMed] [Google Scholar]
- McEvoy RD, Popovic RM, Saunders NA, White DP, 1996. Effects of sustained and repetitive isocapnic hypoxia on ventilation and genioglossal and diaphragmatic EMGs. J. Appl. Physiol 81, 866–875. [DOI] [PubMed] [Google Scholar]
- McEvoy RD, Popovic RM, Saunders NA, White DP, 1997. Ventilatory responses to sustained eucapnic hypoxia in healthy males during wakefulness and NREM sleep.Sleep 20, 1008–1011. [DOI] [PubMed] [Google Scholar]
- McGuire M, Zhang Y, White DP, Ling L, 2003. Chronic intermittent hypoxia enhances ventilatory long-term facilitation in awake rats. J. Appl. Physiol 95, 1499–1508. [DOI] [PubMed] [Google Scholar]
- McIntosh D, Dougherty BJ, 2019. Development of ventilatory long-term facilitation is dependent on estrous cycle stage in adult female rats. Respir. Physiol. Neurobiol 264, 1–7. [DOI] [PubMed] [Google Scholar]
- Millhorn DE, Eldridge FL, Waldrop TG, 1980a. Prolonged stimulation of respiration by a new central neural mechanism. Respir. Physiol 41, 87–103. [DOI] [PubMed] [Google Scholar]
- Millhorn DE, Eldridge FL, Waldrop TG, 1980b. Prolonged stimulation of respiration by endogenous central serotonin. Respir. Physiol 42, 171–188. [DOI] [PubMed] [Google Scholar]
- Mitchell GS, Johnson SM, 2003. Neuroplasticity in respiratory motor control. J. Appl. Physiol 94, 358–374. [DOI] [PubMed] [Google Scholar]
- Mitchell GS, Baker TL, Nanda SA, Fuller DD, Zabka AG, Hodgeman BA,Bavis RW, Mack KJ, Olson EB Jr., 2001a. Invited review: intermittent hypoxiaand respiratory plasticity. J. Appl. Physiol 90, 2466–2475. [DOI] [PubMed] [Google Scholar]
- Mitchell GS, Powell FL, Hopkins SR, Milsom WK, 2001b. Time domains of the hypoxic ventilatory response in awake ducks: episodic and continuous hypoxia. Respir. Physiol 124, 117–128. [DOI] [PubMed] [Google Scholar]
- Moradkhan R, Spitnale B, McQuillan P, Hogeman C, Gray KS, Leuenberger UA,2010. Hypoxia-induced vasodilation and effects of regional phentolamine in awakepatients with sleep apnea. J. Appl. Physiol 108, 1234–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morelli C, Badr MS, Mateika JH, 2004. Ventilatory responses to carbon dioxide at low and high levels of oxygen are elevated after episodic hypoxia in men comparedwith women. J. Appl. Physiol 97, 1673–1680. [DOI] [PubMed] [Google Scholar]
- Morgan BJ, Crabtree DC, Palta M, Skatrud JB, 1995. Combined hypoxia andhypercapnia evokes long-lasting sympathetic activation in humans. J. Appl. Physiol 79, 205–213. [DOI] [PubMed] [Google Scholar]
- Morris KF, Baekey DM, Nuding SC, Dick TE, Shannon R, Lindsey BG, 2003. Invited review: neural network plasticity in respiratory control. J. Appl. Physiol 94, 1242–1252. [DOI] [PubMed] [Google Scholar]
- Muangritdech N, Hamlin MJ, Sawanyawisuth K, Prajumwongs P, Saengjan W,Wonnabussapawich P, Manimmanakorn N, Manimmanakorn A, 2020. Hypoxic training improves blood pressure, nitric oxide and hypoxia-inducible factor-1 alphain hypertensive patients. Eur. J. Appl. Physiol 120, 1815–1826. [DOI] [PubMed] [Google Scholar]
- Mukharliamov F, Smirnova MI, Bedritskii SA, Liadov KV, 2006. Interval hypoxic training in arterial hypertension. Vopr. Kurortol. Fizioter. Lech. Fiz. Kult 5–6. [PubMed] [Google Scholar]
- Narkiewicz K, van de Borne PJ, Pesek CA, Dyken ME, Montano N, Somers VK,1999. Selective potentiation of peripheral chemoreflex sensitivity in obstructive sleep apnea. Circulation 99, 1183–1189. [DOI] [PubMed] [Google Scholar]
- Navarrete-Opazo A, Mitchell GS, 2014. Therapeutic potential of intermittent hypoxia: a matter of dose. Am. J. Phys. Regul. Integr. Comp. Phys 307, R1181–R1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarrete-Opazo A, Alcayaga J, Sepulveda O, Rojas E, Astudillo C, 2017a. Repetitive intermittent hypoxia and Locomotor training enhances walking function in incomplete spinal cord injury subjects: a randomized, triple-blind, Placebo- Controlled Clinical Trial. J. Neurotrauma 34, 1803–1812. [DOI] [PubMed] [Google Scholar]
- Navarrete-Opazo A, Alcayaga JJ, Sepulveda O, Varas G, 2017b. Intermittenthypoxia and Locomotor training enhances dynamic but not standing balance in patients with incomplete spinal cord injury. Arch. Phys. Med. Rehabil 98, 415–424. [DOI] [PubMed] [Google Scholar]
- Nichols NL, Dale EA, Mitchell GS, 2012. Severe acute intermittent hypoxia elicitsphrenic long-term facilitation by a novel adenosine-dependent mechanism. J. Appl. Physiol 112, 1678–1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osanai S, Akiba Y, Fujiuchi S, Nakano H, Matsumoto H, Ohsaki Y, Kikuchi K, 1999. Depression of peripheral chemosensitivity by a dopaminergic mechanism in patients with obstructive sleep apnoea syndrome. Eur. Respir. J 13, 418–423. [DOI] [PubMed] [Google Scholar]
- Ott EP, Baker SE, Holbein WW, Shoemaker JK, Limberg JK, 2019. Effect of varying chemoreflex stress on sympathetic neural recruitment strategies during apnea. J. Neurophysiol 122, 1386–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott EP, Jacob DW, Baker SE, Holbein WW, Scruggs ZM, Shoemaker JK, Limberg JK, 2020. Sympathetic neural recruitment strategies following acute intermittent hypoxia in humans. Am. J. Phys. Regul. Integr. Comp. Phys 318, R961–R971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pamenter ME, Powell FL, 2013. Signalling mechanisms of long term facilitation of breathing with intermittent hypoxia. F1000Prime. Rep 5, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panza GS, Alex RM, Yokhana SS, Lee Pioszak DS, Badr MS, Mateika JH, 2019.Increased oxidative stress, loop gain and the arousal threshold are clinical predictors of increased apnea severity following exposure to intermittent hypoxia. Nat. Sci. Sleep 11, 265–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panza GS, Puri S, Rimar C, Lin H-S, Mateika J, 2020a. Mild intermittent hypoxiaand its multipronged effect on obstructive sleep apnea. FASEB J. 34, 1–1. [Google Scholar]
- Panza GS, Puri S, Rimar C, Lin H-S, Mateika J, 2020b. Mild intermittent hypoxiareduces the critical closing pressure and continuous positive airway pressureresulting in improved treatment adherence. FASEB J. 34, 1–1. [Google Scholar]
- Pawar A, Peng YJ, Jacono FJ, Prabhakar NR, 2008. Comparative analysis of neonatal and adult rat carotid body responses to chronic intermittent hypoxia. J. Appl. Physiol 104, 1287–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng YJ, Prabhakar NR, 2003. Reactive oxygen species in the plasticity of respiratory behavior elicited by chronic intermittent hypoxia. J. Appl. Physiol 94, 2342–2349. [DOI] [PubMed] [Google Scholar]
- Peng YJ, Prabhakar NR, 2004. Effect of two paradigms of chronic intermittent hypoxia on carotid body sensory activity. J. Appl. Physiol 96, 1236–1242. [DOI] [PubMed] [Google Scholar]
- Peng YJ, Overholt JL, Kline D, Kumar GK, Prabhakar NR, 2003. Induction ofsensory long-term facilitation in the carotid body by intermittent hypoxia: implications for recurrent apneas. Proc. Natl. Acad. Sci. U. S. A 100, 10073–10078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pialoux V, Hanly PJ, Foster GE, Brugniaux JV, Beaudin AE, Hartmann SE, Pun M, Duggan CT, Poulin MJ, 2009. Effects of exposure to intermittent hypoxia on oxidative stress and acute hypoxic ventilatory response in humans. Am.J. Respir. Crit. Care Med 180, 1002–1009. [DOI] [PubMed] [Google Scholar]
- Pialoux V, Foster GE, Ahmed SB, Beaudin AE, Hanly PJ, Poulin MJ, 2011. Losartan abolishes oxidative stress induced by intermittent hypoxia in humans.J. Physiol 589, 5529–5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierchala LA, Mohammed AS, Grullon K, Mateika JH, Badr MS, 2008. Ventilatory long-term facilitation in non-snoring subjects during NREM sleep. Respir. Physiol. Neurobiol 160, 259–266. [DOI] [PubMed] [Google Scholar]
- Polkey MI, Harris ML, Hughes PD, Hamnegard CH, Lyons D, Green M,Moxham J, 1997. The contractile properties of the elderly human diaphragm. Am.J. Respir. Crit. Care Med 155, 1560–1564. [DOI] [PubMed] [Google Scholar]
- Prabhakar NR, 2011. Sensory plasticity of the carotid body: role of reactive oxygen species and physiological significance. Respir. Physiol. Neurobiol 178, 375–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakar NR, 2016. Carotid body chemoreflex: a driver of autonomic abnormalities in sleep apnoea. Exp. Physiol 101, 975–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakar NR, Peng YJ, Kumar GK, Nanduri J, Di Giulio C, Lahiri S, 2009. Long-term regulation of carotid body function: acclimatization and adaptation–invited article. Adv. Exp. Med. Biol 648, 307–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puri S, El-Chami M, Shaheen D, Ivers B, Panza GS, Badr MS, Lin HS,Mateika JH, 2020. Variations in loop gain and arousal threshold during NREM sleep are affected by time of day over a 24-hour period in participants with obstructive sleep apnea. J. Appl. Physiol 129, 800–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Querido JS, Godwin JB, Sheel AW, 2008. Intermittent hypoxia reduces cerebrovascular sensitivity to isocapnic hypoxia in humans. Respir. Physiol. Neurobiol 161, 1–9. [DOI] [PubMed] [Google Scholar]
- Querido JS, Rupert JL, McKenzie DC, Sheel AW, 2009. Effects of intermittent hypoxia on the cerebrovascular responses to submaximal exercise in humans. Eur. J. Appl. Physiol 105, 403–409. [DOI] [PubMed] [Google Scholar]
- Querido JS, Kennedy PM, Sheel AW, 2010. Hyperoxia attenuates muscle sympathetic nerve activity following isocapnic hypoxia in humans. J. Appl. Physiol 108, 906–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Querido JS, Welch JF, Ayas NT, Sheel AW, 2015. Cerebrovascular response toCO2 following 10 days of intermittent hypoxia in humans. Aerosp. Med. Hum. Perform 86, 782–786. [DOI] [PubMed] [Google Scholar]
- Rey S, Del RR, Alcayaga J, Iturriaga R, 2004. Chronic intermittent hypoxia enhances cat chemosensory and ventilatory responses to hypoxia. J. Physiol 560, 577–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley KL, Mantilla CB, Sieck GC, 2005. Respiratory muscle plasticity. Respir. Physiol. Neurobiol 147, 235–251. [DOI] [PubMed] [Google Scholar]
- Rowley JA, Deebajah I, Parikh S, Najar A, Saha R, Badr MS, 2007. The influence of episodic hypoxia on upper airway collapsibility in subjects with obstructive sleep apnea. J. Appl. Physiol 103, 911–916. [DOI] [PubMed] [Google Scholar]
- Roy A, Farnham MMJ, Derakhshan F, Pilowsky PM, Wilson RJA, 2018. Acuteintermittent hypoxia with concurrent hypercapnia evokes P2X and TRPV1 receptor- dependent sensory long-term facilitation in naïve carotid bodies. J. Physiol 596, 3149–3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeed O, Bhatia V, Formica P, Browne A, Aldrich TK, Shin JJ, Maybaum S, 2012. Improved exercise performance and skeletal muscle strength after simulated altitude exposure: a novel approach for patients with chronic heart failure. J. Card. Fail 18, 387–391. [DOI] [PubMed] [Google Scholar]
- Sankari A, Bascom AT, Riehani A, Badr MS, 2015. Tetraplegia is associated with enhanced peripheral chemoreflex sensitivity and ventilatory long-term facilitation.J. Appl. Physiol 119, 1183–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilero GJ, Spungen AM, Bauman WA, Radulovic M, Lesser M, 2009. Pulmonary function and spinal cord injury. Respir. Physiol. Neurobiol 166, 129–141. [DOI] [PubMed] [Google Scholar]
- Scott BR, Slattery KM, Dascombe BJ, 2014. Intermittent hypoxic resistance training: does it provide added benefit? Front. Physiol 5, 397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serebrovska TV, Portnychenko AG, Drevytska TI, Portnichenko VI, Xi L, Egorov E, Gavalko AV, Naskalova S, Chizhova V, Shatylo VB, 2017. Intermittent hypoxia training in prediabetes patients: beneficial effects on glucose homeostasis, hypoxia tolerance and gene expression. Exp. Biol. Med. (Maywood) 242, 1542–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serebrovskaya TV, Manukhina EB, Smith ML, Downey HF, Mallet RT, 2008. Intermittent hypoxia: cause of or therapy for systemic hypertension? Exp. Biol. Med. (Maywood) 233, 627–650. [DOI] [PubMed] [Google Scholar]
- Shah R, Wong V, Robinson D, Fletcher H, Estrada L, Moxham J, Rafferty G,Harridge S, Lazarus N, Jolley C, 2019. P186 the effect of healthy ageing on human phrenic nerve function. Thorax 74, A190–A191. [Google Scholar]
- Shatilo VB, Korkushko OV, Ischuk VA, Downey HF, Serebrovskaya TV, 2008.Effects of intermittent hypoxia training on exercise performance, hemodynamics, and ventilation in healthy senior men. High Alt. Med. Biol 9, 43–52. [DOI] [PubMed] [Google Scholar]
- Shkoukani M, Babcock MA, Badr MS, 2002. Effect of episodic hypoxia on upper airway mechanics in humans during NREM sleep. J. Appl. Physiol 92, 2565–2570. [DOI] [PubMed] [Google Scholar]
- Somers VK, Mark AL, Abboud FM, 1991. Interaction of baroreceptor andchemoreceptor reflex control of sympathetic nerve activity in normal humans. J. Clin. Invest 87, 1953–1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner S, Schueller PO, Schulze V, Strauer BE, 2010. Occurrence of coronary collateral vessels in patients with sleep apnea and total coronary occlusion. Chest 137, 516–520. [DOI] [PubMed] [Google Scholar]
- Stipica I, Pavlinac Dodig I, Pecotic R, Dogas Z, Valic Z, Valic M, 2016. Periodicity during hypercapnic and hypoxic stimulus is crucial in distinct aspects of phrenic nerve plasticity. Physiol. Res 65, 133–143. [DOI] [PubMed] [Google Scholar]
- Stuckless TJR, Vermeulen TD, Brown CV, Boulet LM, Shafer BM, Wakeham DJ, Steinback CD, Ayas NT, Floras JS, Foster GE, 2020. Acute intermittenthypercapnic hypoxia and sympathetic neurovascular transduction in men. J. Physiol 598, 473–487. [DOI] [PubMed] [Google Scholar]
- Sun X, Deng J, Liu T, Borjigin J, 2002. Circadian 5-HT production regulated by adrenergic signaling. Proc. Natl. Acad. Sci. U. S. A 99, 4686–4691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed Z, Lin HS, Mateika JH, 2013. The impact of arousal state, sex, and sleep apneaon the magnitude of progressive augmentation and ventilatory long-termfacilitation. J. Appl. Physiol 114, 52–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamisier R, Nieto L, Anand A, Cunnington D, Weiss JW, 2004. Sustained muscle sympathetic activity after hypercapnic but not hypocapnic hypoxia in normalhumans. Respir. Physiol. Neurobiol 141, 145–155. [DOI] [PubMed] [Google Scholar]
- Tamisier R, Anand A, Nieto LM, Cunnington D, Weiss JW, 2005. Arterial pressure and muscle sympathetic nerve activity are increased after two hours of sustained but not cyclic hypoxia in healthy humans. J. Appl. Physiol 98, 343–349. [DOI] [PubMed] [Google Scholar]
- Tamisier R, Pepin JL, Remy J, Baguet JP, Taylor JA, Weiss JW, Levy P, 2011.14 nights of intermittent hypoxia elevate daytime blood pressure and sympatheticactivity in healthy humans. Eur. Respir. J 37, 119–128. [DOI] [PubMed] [Google Scholar]
- Tan H, Lu H, Chen Q, Tong X, Jiang W, Yan H, 2018. The effects of intermittentwhole-body hypoxic preconditioning on patients with carotid artery stenosis. WorldNeurosurg. 113, e471–e479. [DOI] [PubMed] [Google Scholar]
- Taralov ZZ, Terziyski KV, Dimov PK, Marinov BI, Kostianev SS, 2018. Assessment of the impact of 10-day intermittent hypoxia on the autonomic controlmeasured by heart rate variability. Physiol. Int 105, 386–396. [DOI] [PubMed] [Google Scholar]
- Tester NJ, Fuller DD, Fromm JS, Spiess MR, Behrman AL, Mateika JH, 2014.Long-term facilitation of ventilation in humans with chronic spinal cord injury. Am.J. Respir. Crit. Care Med 189, 57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjong YW, Chen Y, Liong EC, Tipoe GL, Fung ML, 2006. Chronic hypoxia modulates the function and expression of melatonin receptors in the rat carotidbody. J. Pineal Res 40, 125–134. [DOI] [PubMed] [Google Scholar]
- Tobin B, Costalat G, Renshaw GMC, 2020. Intermittent not continuous hypoxia provoked haematological adaptations in healthy seniors: hypoxic pattern may hold the key. Eur. J. Appl. Physiol 120, 707–718. [DOI] [PubMed] [Google Scholar]
- Tolep K, Higgins N, Muza S, Criner G, Kelsen SG, 1995. Comparison of diaphragm strength between healthy adult elderly and young men. Am. J. Respir. Crit. Care Med 152, 677–682. [DOI] [PubMed] [Google Scholar]
- Trumbower RD, Jayaraman A, Mitchell GS, Rymer WZ, 2012. Exposure to acute intermittent hypoxia augments somatic motor function in humans with incomplete spinal cord injury. Neurorehabil. Neural Repair 26, 163–172. [DOI] [PubMed] [Google Scholar]
- Trumbower RD, Hayes HB, Mitchell GS, Wolf SL, Stahl VA, 2017. Effects of acute intermittent hypoxia on hand use after spinal cord trauma: a preliminarystudy. Neurology 89, 1904–1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tun Y, Hida W, Okabe S, Kikuchi Y, Kurosawa H, Tabata M, Shirato K, 2000. Effects of nasal continuous positive airway pressure on awake ventilatory responsesto hypoxia and hypercapnia in patients with obstructive sleep apnea. Tohoku J. Exp. Med 190, 157–168. [DOI] [PubMed] [Google Scholar]
- Turner DL, Mitchell GS, 1997. Long-term facilitation of ventilation following repeated hypoxic episodes in awake goats. J. Physiol 499, 543–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valic M, Pecotic R, Pavlinac Dodig I, Valic Z, Stipica I, Dogas Z, 2016. Intermittent hypercapnia-induced phrenic long-term depression is revealed afterserotonin receptor blockade with methysergide in anaesthetized rats. Exp. Physiol 101, 319–331. [DOI] [PubMed] [Google Scholar]
- Velotta JP, Cheviron ZA, 2018. Remodeling ancestral phenotypic plasticity in localadaptation: a new framework to explore the role of genetic compensation in theevolution of homeostasis. Integr. Comp. Biol 58, 1098–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeulen TD, Benbaruj J, Brown CV, Shafer BM, Floras JS, Foster GE, 2020.Peripheral chemoreflex contribution to ventilatory long-term facilitation induced by acute intermittent hypercapnic hypoxia in males and females. J. Physiol 598, 4713–4730. [DOI] [PubMed] [Google Scholar]
- Viscor G, Torrella JR, Corral L, Ricart A, Javierre C, Pages T, Ventura JL, 2018.Physiological and biological responses to short-term intermittent hypobaric hypoxiaexposure: from sports and mountain medicine to new biomedical applications. Front.Physiol 9, 814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivodtzev I, Tan AQ, Hermann M, Jayaraman A, Stahl V, Rymer WZ, Mitchell GS, Hayes HB, Trumbower RD, 2020. Mild to moderate sleep apnea islinked to hypoxia-induced motor recovery after spinal cord injury. Am. J. Respir. Crit. Care Med 202, 887–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadhwa H, Gradinaru C, Gates GJ, Badr MS, Mateika JH, 2008. Impact of intermittent hypoxia on long-term facilitation of minute ventilation and heart ratevariability in men and women: do sex differences exist? J. Appl. Physiol 104, 1625–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlin-Larsson B, Ulfberg J, Aulin KP, Kadi F, 2009. The expression of vascular endothelial growth factor in skeletal muscle of patients with sleep disorders. Muscle Nerve 40, 556–561. [DOI] [PubMed] [Google Scholar]
- Wang H, Shi X, Schenck H, Hall JR, Ross SE, Kline GP, Chen S, Mallet RT, Chen P, 2020. Intermittent hypoxia training for treating mild cognitive impairment: a pilot study. Am. J. Alzheimers Dis. Other Dement 35, 1533317519896725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wecht JM, Harel NY, Guest J, Kirshblum SC, Forrest GF, Bloom O, Ovechkin AV, Harkema S, 2020. Cardiovascular autonomic dysfunction in spinalcord injury: epidemiology, diagnosis, and management. Semin. Neurol 40, 550–559. [DOI] [PubMed] [Google Scholar]
- Weil JV, 1986. Ventilatory control at high altitude. Handbook of Physiology, Section 3:The Respiratory System-Vol II: Control of Breathing, Part 2. American Physiological Society, Bethesda, MD, pp. 703–728. [Google Scholar]
- Wilkerson JER, Devinney M, Mitchell GS, 2018. Intermittent but not sustainedmoderate hypoxia elicits long-term facilitation of hypoglossal motor output. Respir. Physiol. Neurobiol 256, 15–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams AM, Gee CM, Voss C, West CR, 2019. Cardiac consequences of spinalcord injury: systematic review and meta-analysis. Heart 105, 217–225. [DOI] [PubMed] [Google Scholar]
- Xie A, Skatrud JB, Puleo DS, Morgan BJ, 2001. Exposure to hypoxia produces long-lasting sympathetic activation in humans. J. Appl. Physiol 91, 1555–1562. [DOI] [PubMed] [Google Scholar]
- Xing T, Fong AY, Bautista TG, Pilowsky PM, 2013. Acute intermittent hypoxia induced neural plasticity in respiratory motor control. Clin. Exp. Pharmacol. Physiol 40, 602–609. [DOI] [PubMed] [Google Scholar]
- Yang C, Zhang L, Hao H, Ran M, Li J, Dong H, 2019. Serotonergic neurons in the dorsal raphe nucleus mediate the arousal-promoting effect of orexin during isoflurane anesthesia in male rats. Neuropeptides 75, 25–33. [DOI] [PubMed] [Google Scholar]
- Yokhana SS, Gerst III DG, Lee DS, Badr MS, Qureshi T, Mateika JH, 2012. Impact of repeated daily exposure to intermittent hypoxia and mild sustainedhypercapnia on apnea severity. J. Appl. Physiol 112, 367–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabka AG, Behan M, Mitchell GS, 2001a. Long term facilitation of respiratory motoroutput decreases with age in male rats. J. Physiol 531, 509–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabka AG, Behan M, Mitchell GS, 2001b. Selected contribution: time-dependenthypoxic respiratory responses in female rats are influenced by age and by the estrus cycle. J. Appl. Physiol 91, 2831–2838. [DOI] [PubMed] [Google Scholar]
- Zabka AG, Mitchell GS, Behan M, 2006. Conversion from testosterone to oestradiol is required to modulate respiratory long-term facilitation in male rats. J. Physiol 576, 903–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Downey HF, Shi X, 2010. Acute intermittent hypoxia exposures enhance arterial oxygen delivery. Exp. Biol. Med. (Maywood) 235, 1134–1141. [DOI] [PubMed] [Google Scholar]
- Zhang P, Downey HF, Chen S, Shi X, 2014. Two-week normobaric intermittenthypoxia exposures enhance oxyhemoglobin equilibrium and cardiac responses during hypoxemia. Am. J. Phys. Regul. Integr. Comp. Phys 307, R721–R730. [DOI] [PubMed] [Google Scholar]
- Zhang P, Shi X, Downey HF, 2015. Two-week normobaric intermittent-hypoxic exposures stabilize cerebral perfusion during hypocapnia and hypercapnia. Exp. Biol. Med. (Maywood) 240, 961–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou XS, Shahabuddin S, Zahn BR, Babcock MA, Badr MS, 2000. Effect of gender on the development of hypocapnic apnea/hypopnea during NREM sleep. J. Appl. Physiol 89, 192–199. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.