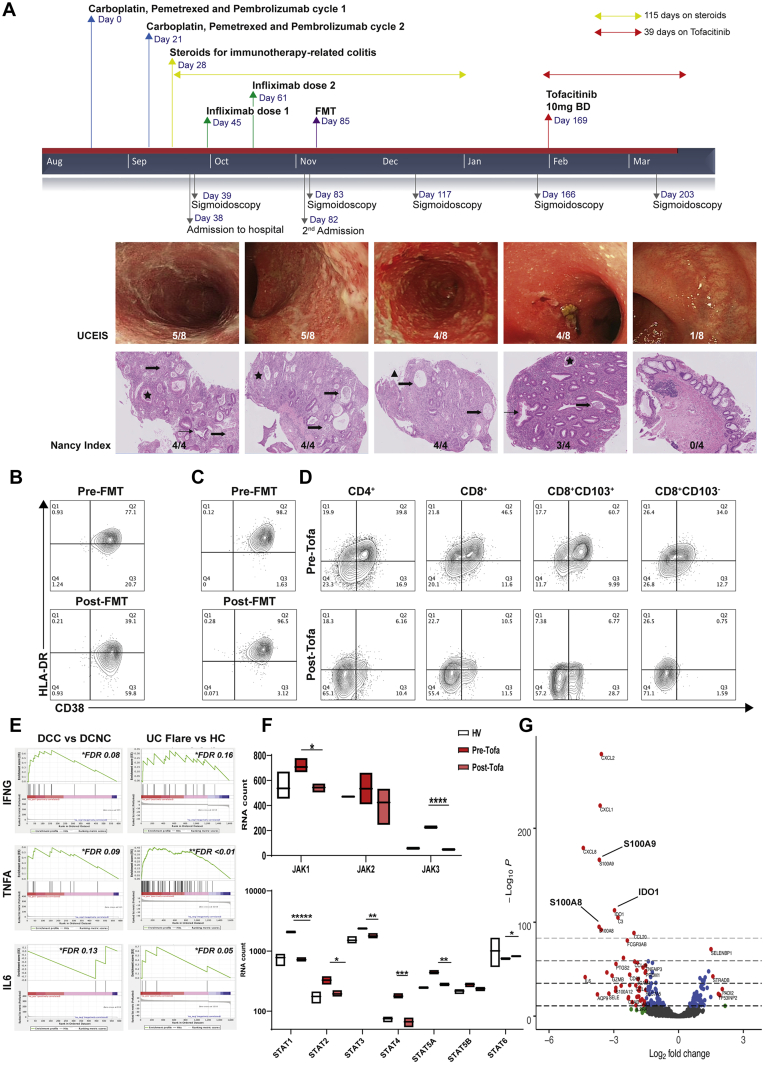
Figure 7.
Tofacitinib results in rapid resolution of treatment-refractory ICI-colitis, and correlates with resolution of CD8+ TRM cell activation and down-regulation of JAK/STAT signaling. (A) Clinical time course of a 61-year-old man with non–small-cell lung cancer treated with carboplatin, pemetrexed, and pembrolizumab. The anti–PD-1 colitis was refractory to multiple therapies. Tofacitinib resulted in prompt resolution of clinical symptoms, and endoscopic and histopathology inflammation. Tofacitinib was continued for 6 weeks. Star, crypt abscess; thick arrow, attenuated crypt; thin arrow, crypt architectural distortion; triangle, erosion. (B) FMT response in a previous patient with ICI-colitis, where clinical resolution was associated with normalization of CD8+ TRM cell activation. Flow cytometry gated on live CD3+CD8+CD69+CD103+ TRM cells. (C) Using the same donor stool, FMT did not result in resolution of clinical symptoms or resolution of CD8+ TRM cell activation in this 61-year-old man who subsequently received tofacitinib. (D) Flow cytometry plots gated on live single CD45+CD3+ T cells are shown. Before tofacitinib, widespread activation of CD4+ and CD8+ T cells is evident, with the highest level of activation in CD8+CD103+ TRM cell subset (61%). After 6 weeks of tofacitinib, there is resolution of T cell activation, including in the CD8+CD103+ TRM cell subset (7%). (E) Gene set enrichment analysis of bulk RNASeq data demonstrates IFNG signaling pathway enrichment in ICI-colitis. (F) Data from Nanostring RNAplex assay from the tofacitinib-treated patient and 3 HVs. Tofacitinib results in significant down-regulation of JAK1, JAK3, STAT1, STAT2, STAT3, STAT4, and STAT5A. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001; and ∗∗∗∗∗P < .00001 by Mann-Whitney test. (G) Volcano plot depicting pre and post tofacitinib.