Figure 2. Receptor blocking and structural studies.
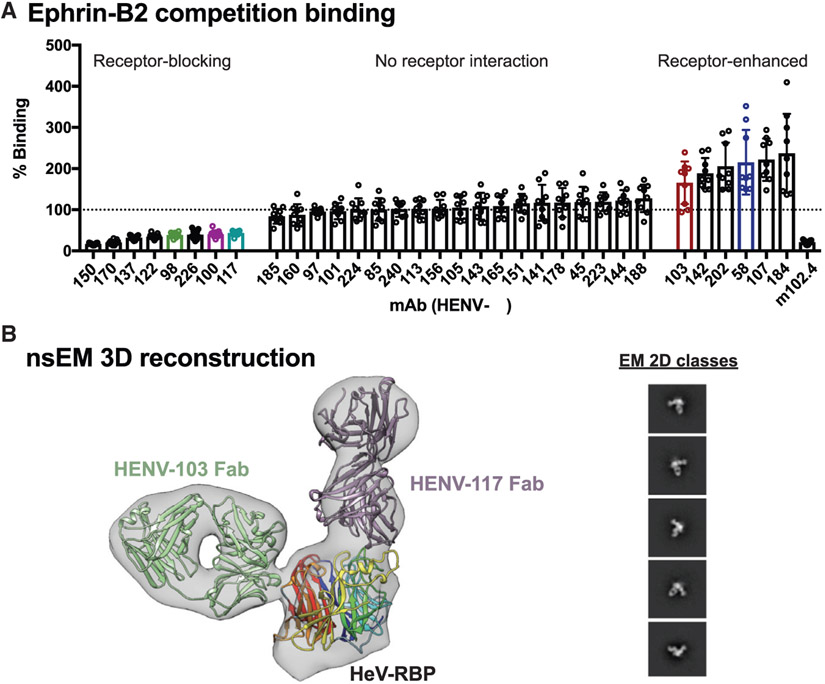
(A) Antibody binding to cell surface-displayed HeV-RBP when ephrin-B2 is bound. Cells transiently transfected with a cDNA encoding the full-length HeV-RBP were incubated with a saturating concentration of recombinantly expressed ephrin-B2. Without washing, cells were incubated with 2 μg/mL antibody, and binding was compared to binding of antibodies in the absence of ephrin-B2. The mAb m102.4 served as a control for receptor competition. Pooled data from three independent experiments are shown. Data are represented by mean ± SD.
(B) Three-dimensional reconstruction from negative stain electron microscopy (nsEM) of dimeric HeV-RBP full ectodomain bound to HENV-103 Fab and HENV-117 Fab. The EM map is shown in gray, the Fabs are in purple and green, and the RBP head domain is colored by β-propeller. 2D classes are shown, with box size of 128 at Å per pixel (Å/pix) of 3.5.