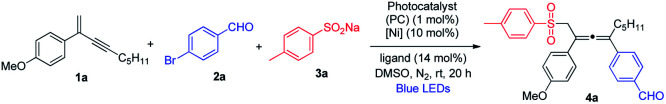
Optimization of the 1,4-sulfonylarylation reaction conditionsa.
| ||||
|---|---|---|---|---|
| Entry | PC | [Ni] | Ligand | Yieldb (%) |
| 1 | Ru(bpy)3Cl2·6H2O | NiCl2·glyme | dtbbpy | 55 |
| 2 | Ru(bpy)3(PF6)2 | NiCl2·glyme | dtbbpy | 45 |
| 3 | [Ir(dFCF3ppy)2(dtbbpy)]PF6 | NiCl2·glyme | dtbbpy | 62 |
| 4 | 4CzIPN | NiCl2·glyme | dtbbpy | 64 |
| 5 | 4CzIPN | NiCl2 | dtbbpy | 35 |
| 6 | 4CzIPN | NiCl2·6H2O | dtbbpy | 11 |
| 7 | 4CzIPN | NiCl2·glyme | diOMebpy | 73 |
| 8 | 4CzIPN | NiCl2·glyme | bpy | 62 |
| 9 | 4CzIPN | NiCl2·glyme | Phen | 0 |
| 10c | 4CzIPN | NiCl2·glyme | diOMebpy | 72 |
| 11 | — | NiCl2·glyme | diOMebpy | 0 |
| 12 | 4CzIPN | — | diOMebpy | 0 |
| 13 | 4CzIPN | NiCl2·glyme | — | 0 |
| 14d | 4CzIPN | NiCl2·glyme | diOMebpy | 0 |
| 15e | 4CzIPN | NiCl2·glyme | diOMebpy | 0 |
Reaction conditions: 1a (0.2 mmol), 2a (0.1 mmol) and 3a (0.12 mmol) in DMSO (1.0 mL), photocatalyst (1 mol%), nickel-precatalyst (10 mol%), ligand (14 mol%), at room temperature, 30 W blue LEDs, 20 h.
Isolated yield.
NiCl2·glyme (5 mol%), diOMebpy (7 mol%).
No light.
Reaction was performed in air.
