Fig. 1.
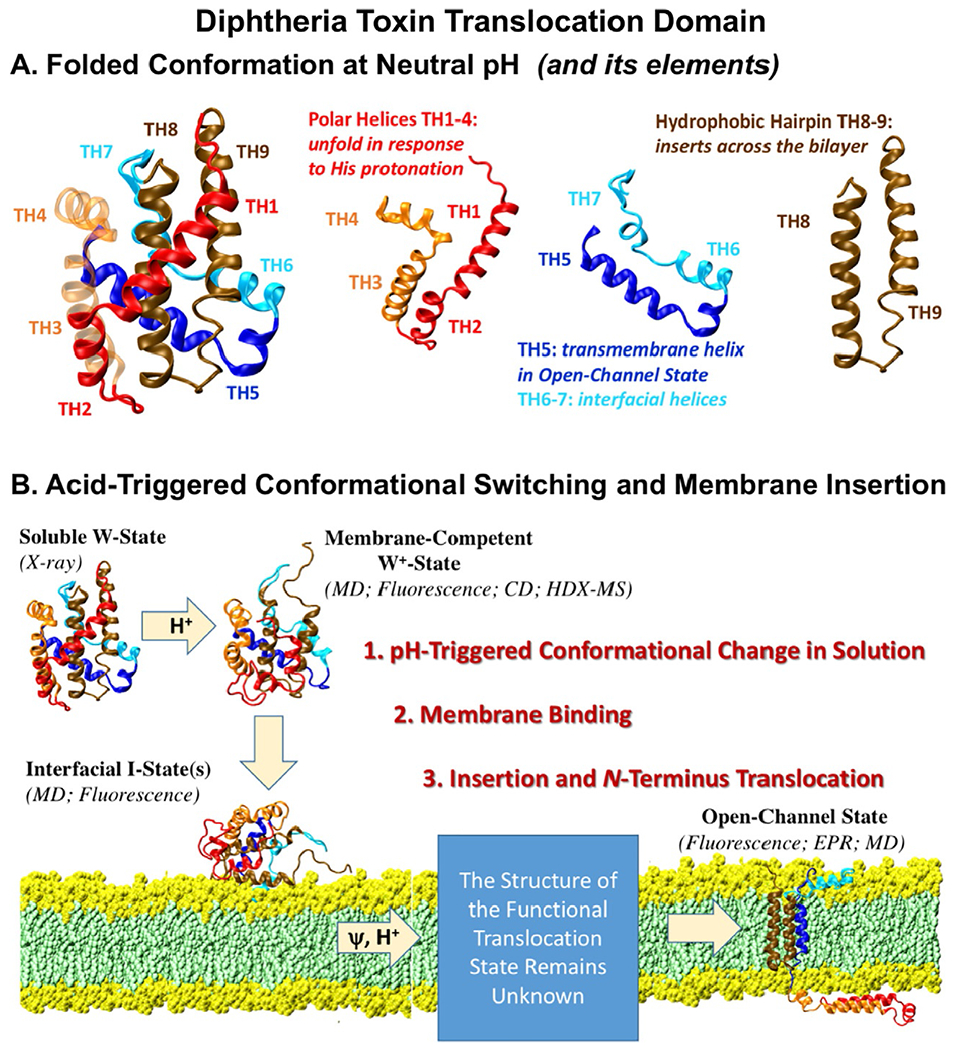
(A) Crystallographic structure of the diphtheria toxin translocation domain [Bennett & Eisenberg, 1994; Weiss et al., 1995] consists of several structural elements that undergo conformational changes in response to protonation and membrane interactions. (B) pH-triggered membrane insertion pathway of the diphtheria toxin T-domain [Kurnikov et al., 2013; Kyrychenko, Posokhov, Rodnin, & Ladokhin, 2009], responsible for the cellular entry of the toxin.
