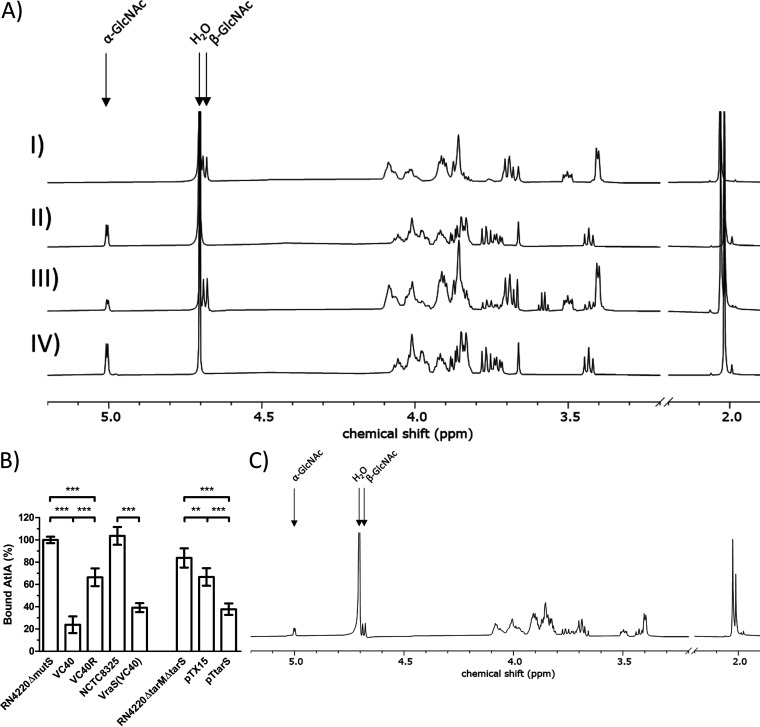
FIG 4.
(A) 1H NMR spectra of purified WTA monomers from S. aureus RN4220ΔtarMΔtarS pTtarS (I), S. aureus RN4220ΔmutS (II), S. aureus VC40 (III), and S. aureus VC40R (IV). Arrows indicate the 1H proton of the α-1,4-GlcNAc and β-1,4-GlcNAc residues on ribitol phosphate and the water peak, respectively. The figure shows a representative experiment; the analysis was performed in triplicate. (B) AtlA binding assay to determine the amount of bound AtlA on PGN with WTA after the removal of d-alanine substitutions. S. aureus RN4220ΔmutS set to 100%. (*, P < 0.05; **, P < 0.005; ***, P < 0.0005). (C) 1H NMR spectrum of purified WTA monomers from S. aureus VC40R grown in medium with 4% NaCl. Arrows indicate the 1H proton of the α-1,4-GlcNAc and β-1,4-GlcNAc residues on ribitol phosphate and the water peak, respectively. The figure shows a representative experiment, and the analysis was performed in triplicate.