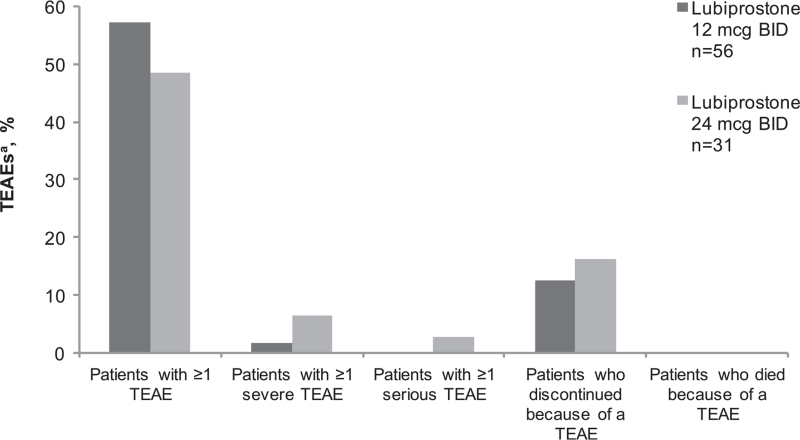
FIGURE 1.
Overview of treatment-emergent adverse events (safety population). aTEAE is any event with an onset date on or after the first dose of study medication and with an onset date no more than 7 days after the last dose of study medication. BID = twice daily; n = subgroup of population; TEAE = treatment-emergent adverse event.