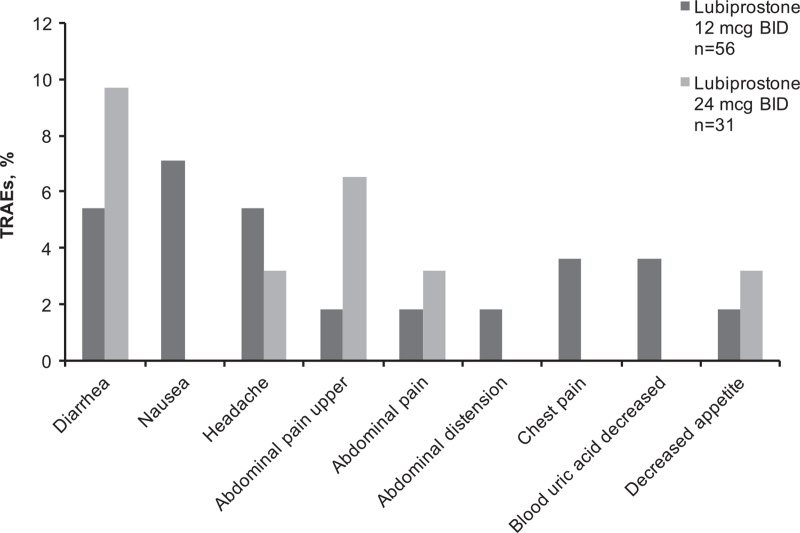
FIGURE 2.
Treatment-related adverse events reported by ≥5% of patients or GI disorders reported in ≥3% of patients in either lubiprostone treatment group (safety population). GI disorders: diarrhea, nausea, upper abdominal pain, abdominal pain, abdominal distension. BID = twice daily; GI = gastrointestinal; n = subgroup of population; TRAE = treatment-related adverse event.