Yields for protected triazoles 12a–g from the copper azide-alkyne cyclisation coupling of 5′-O-propargyluridine (8) and α-azidophosphonates (11a–g), followed by their deprotection to form the final deprotected 1,2,3-triazole series in their Na+ salt form (13a–g).
| R1 | Click yield (R2: OBn)a | Deprotection yield (R2: O−Na+)b |
|---|---|---|
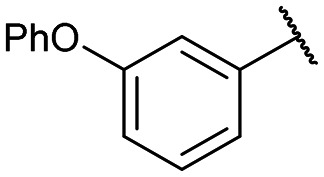
|
12a: 51% | 13a-(s): 99% |
| 13a-(l): 91% | ||
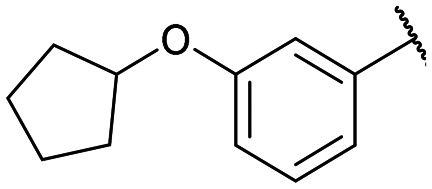
|
12b: 52% | 13b-(s): 94% |
| 13b-(l): 99% | ||
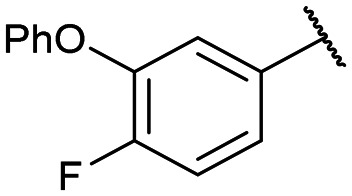
|
12c: 66% | 13c-(s): 97% |
| 13c-(l): 91% | ||
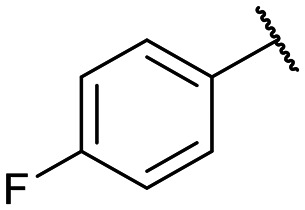
|
12d: 57% | 13d-(s): 92% |
| 13d-(l): 62% | ||
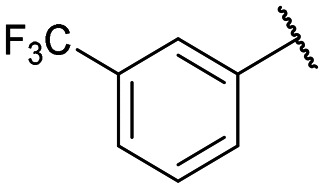
|
12e: 60% | 13e-(s): 92% |
| 13e-(l): 85% | ||
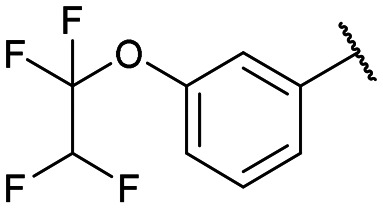
|
12f: 52% | 13f-(s): 95% |
| 13f-(l): 92% | ||
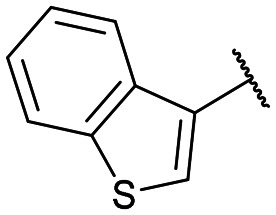
|
12g: 57% | 13g-(s): 96% |
| 13g-(l): 92% |
