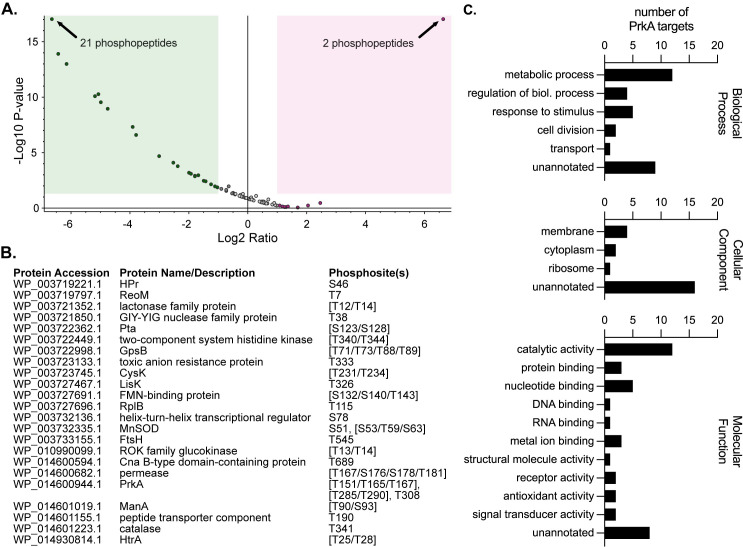
Fig 1. PrkA phosphorylates proteins involved in diverse processes in the context of cell wall stress.
(A) Volcano plot showing log2 fold-change of abundance ratios versus -log10 P value of phosphorylated peptides with XCorr scores > 2.0 in the ΔprkA::erm mutant compared to wild type. The green box highlights peptides that were two-fold or less abundant and significantly different (P < 0.05) and the red box highlights peptides that were two-fold or more abundant and significantly different in the ΔprkA::erm mutant; some dots represent more than one phosphopeptide (indicated). (B) List of proteins containing phosphosites that were significantly less abundant in the ΔprkA::erm mutant compared to wild type. Differentially phosphorylated peptides from (A) that differed only by oxidations, deamidations, and/or missed cleavages were combined into the same phosphosite. Amino acids in brackets indicate ambiguous phosphosites. (C) GO term analysis of the proteins from (B). Some proteins are listed in more than one annotation per graph.