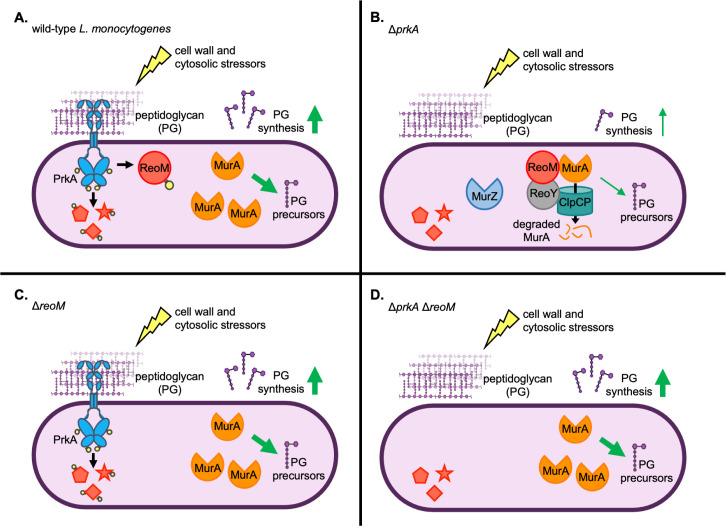
Fig 7. Model: PrkA-mediated regulation of ReoM is required for peptidoglycan synthesis control during cell wall stress and in the cytosol.
(A) In wild-type L. monocytogenes, the PASTA kinase PrkA monitors cell wall integrity by sensing peptidoglycan (PG) perturbations. PrkA autophosphorylates to become activated (yellow circles indicate phosphorylations) by cell wall-targeting and cytosolic stressors and in turn phosphorylates substrates (represented by red shapes with yellow circles) that promote adaptation to cell wall stress and to the cytosol. Specifically, PrkA phosphorylates ReoM, which stabilizes the essential enzyme MurA by preventing targeting of MurA to the ClpCP protease. Increased MurA levels increases production of PG precursors, leading to increased PG synthesis. (B) In a ΔprkA mutant, PrkA substrates (red shapes) remain unphosphorylated in the presence of cell wall-targeting or cytosolic stressors. Unphosphorylated ReoM promotes ClpCP-dependent degradation of MurA and prevents an increase in PG synthesis. ReoY and MurZ also contribute to controlling MurA levels. Molecular interactions shown between ReoM, ReoY, MurA, and ClpCP are based on the findings of Wamp et al. [52]. (C) In a ΔreoM mutant, the absence of ReoM stabilizes MurA levels and increases PG synthesis (even in the absence of stress, not shown). PrkA can also phosphoregulate its other downstream targets in the presence of cell wall-targeting or cytosolic stressors. (D) In a ΔprkA ΔreoM mutant, lack of ReoM stabilizes MurA and leads to increased PG synthesis (even in the absence of stress, not shown), but other PrkA substrates cannot be phosphorylated during cell wall or cytosolic stress.