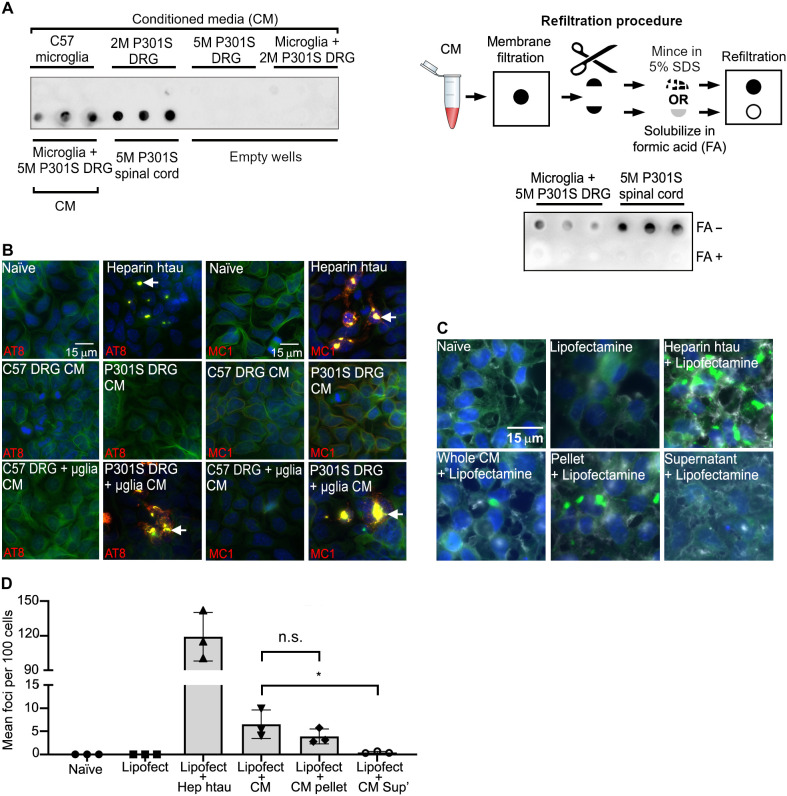
Fig. 2. Microglia cocultured with 5M P301S DRGn release insoluble tau aggregates capable of seeding.
(A) Insoluble tau in the CM is captured on a filter trap (detected with the anti–htau antibody HT7). Top left filter, top row: No tau in CM from monocultures of DRGn (5M C57 and 2M P301S, which do not contain tau aggregates, and 5M P301S) or cocultures of C57 microglia with 2M P301S DRGn. Top left filter, bottom row: htau in CM from cocultures of microglia with 5M P301S DRGn, with spinal cord extracts as positive controls. Bottom right filter: Refiltration of captured tau solubilized in formic acid (FA+) leads to loss of signal, but minced samples (FA−) retain signal. A scheme of the refiltration procedure is also shown (top right). (B) Tau in CM seeds new aggregates in the reporter system HEK-P301S-venus cells. The tau-seeded aggregates contain hyperphosphorylated and conformationally altered tau as shown by AT8 and MC1 antibody staining (red). Alexa Fluor-647–conjugated phalloidin (green) was used to outline the cell perimeter. Nuclei were stained by DAPI (blue). (C and D) Aggregated P301S-venus foci are obtained with the 100,000g pellet but not the supernatant of CM after ultracentrifugation (P = 0.0429, Friedman test). Representative images and quantification of fractionated CM seeded aggregation in HEK-P301S-venus cells. Lipofectamine was added to increase the efficiency of the uptake. The number of aggregates per 100 cells is quantified in (D). N = 3 independent cultures of neurons used for CM preparations, means ± SD.