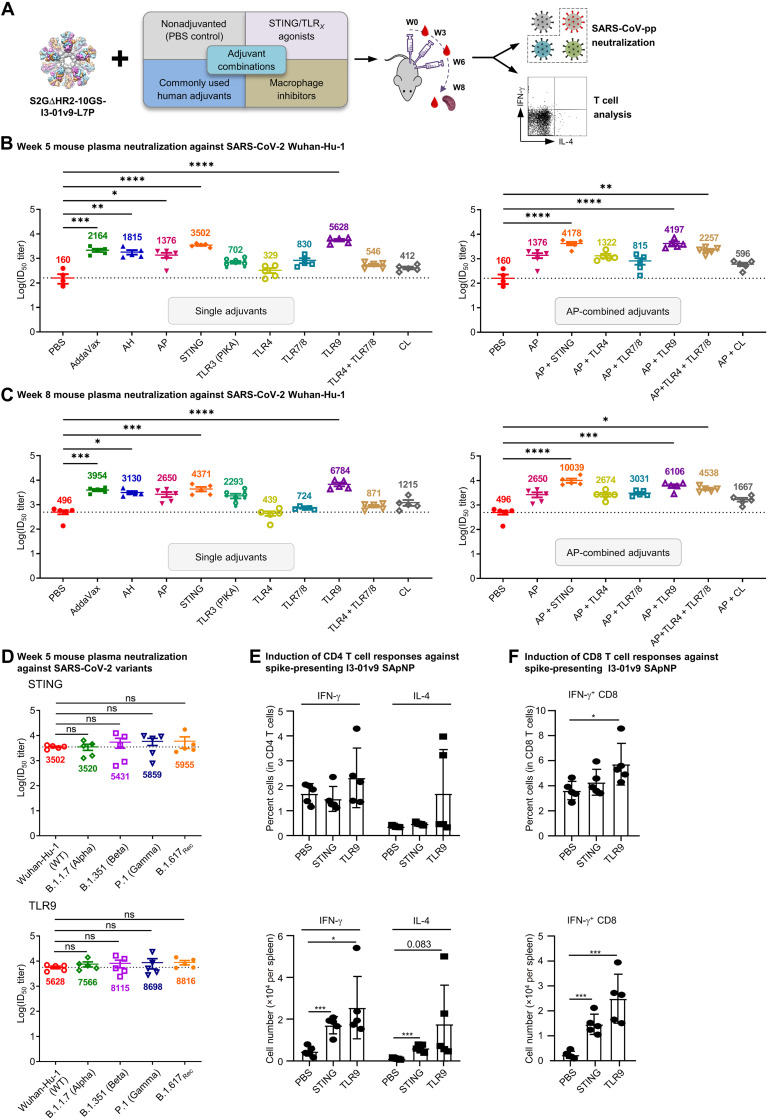
Fig. 2. Adjuvants enhance the I3-01v9 SApNP vaccine-induced plasma neuralization of both the wild-type strain and four variants.
(A) Schematic representation of mouse immunization with the I3-01v9 SApNP with diverse adjuvant formulations and functional assessment by SARS-CoV-2-pp neutralization assays and T cell analysis. Conventional adjuvants, STING/TLR agonists, macrophage inhibitors, and adjuvant combinations were compared to nonadjuvanted control [phosphate-buffered saline (PBS)]. (B and C) Mouse plasma neutralization against the wild-type SARS-CoV-2 strain, Wuhan-Hu-1, at weeks 5 and 8 after two and three footpad injections, respectively. ID50 titers derived from SARS-CoV-2-pp neutralization assays are plotted, with average ID50 values labeled on the plots. (D) Neutralization against four variants by mouse plasma from STING (top)– and CpG (bottom)–formulated vaccine groups. ID50 titers derived from SARS-CoV-2-pp neutralization assays are plotted. Neutralization data were analyzed using either one-way ANOVA (B and C) or repeated measures one-way ANOVA (D) to compare ID50 titers. Dunnett’s multiple comparison post hoc test was performed. Splenic mononuclear cells derived from mice in the STING and CpG groups (n = 5 mice per group) at week 8 were cultured in the presence of BALB/C DCs pulsed with I3-01v9 SApNP (1 × 10−7 mM). Cells were harvested 16 hours following reactivation. (E) Production of IFN-γ–producing TH1 CD4+ T cells and IL-4–producing TH2 CD4+ T cells. (F) IFN-γ–producing CD8+ effector T cells. T cell responses were analyzed using one-way ANOVA followed by Tukey’s multiple comparison post hoc test. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001.