Figure 5.
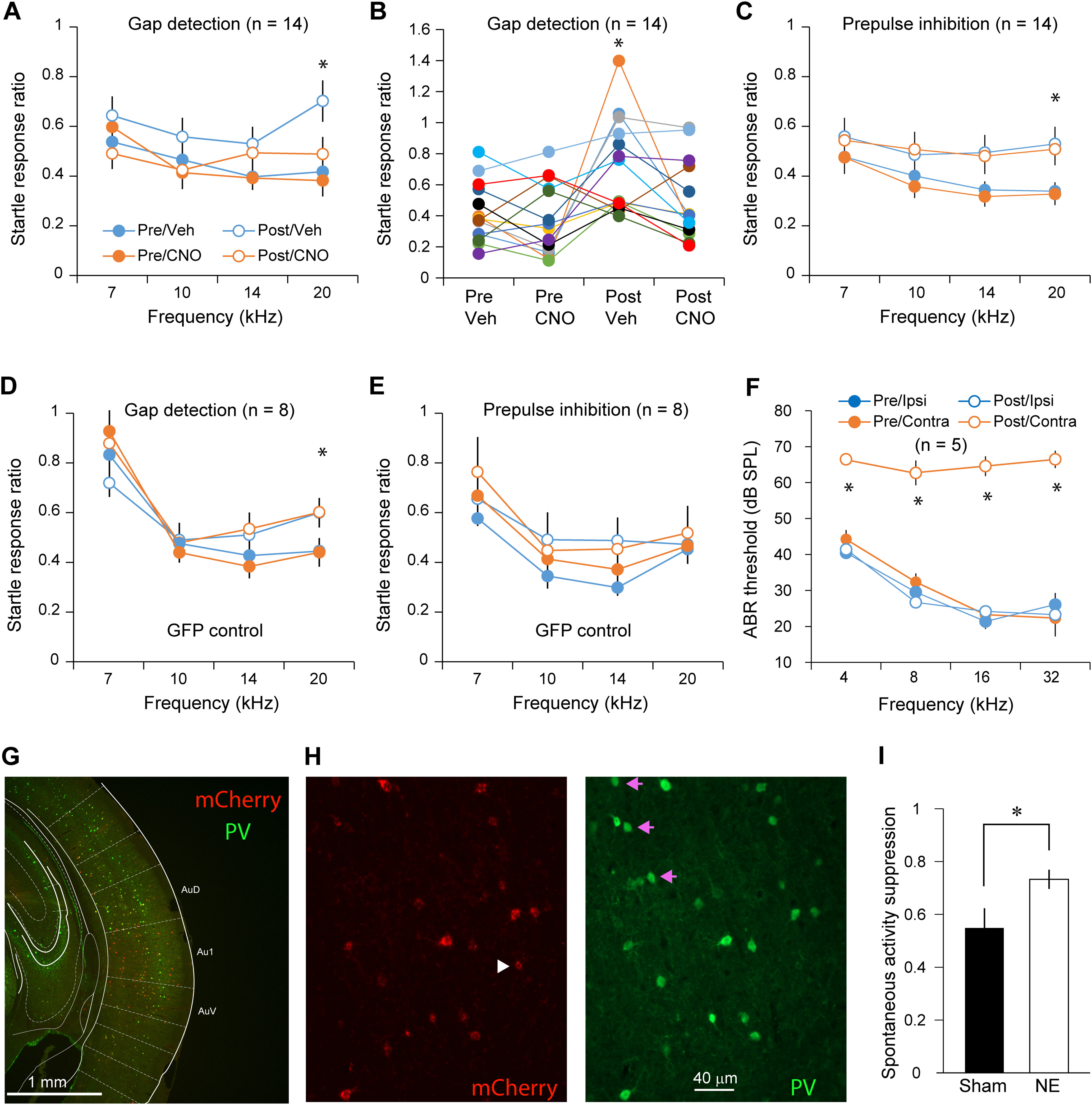
Chemogenetic activation of PV neurons reversed impairments in gap detection in noise-exposed animals. A, Noise exposure significantly impaired gap detection performance. CNO injection in mice with hM3D expression in PV neurons completely reversed the impairments in gap detection after hearing lesions but did not change behavior before hearing lesions. B, Gap detection performance from individual mice at 20 kHz. C, Noise exposure reduces the effect of prepulse inhibition on the startle response ratio. CNO injection did not have an impact on behavior. All mice were tested before and after CNO injections. D, CNO did not alter gap detection performance in mice with GFP expression in PV neurons. E, CNO did not alter PPI performance in mice with GFP expression in PV neurons. F, ABR thresholds were significantly greater only in the left ear 10 d after hearing lesions; * indicates a statistically significant difference for the frequency bin and between the treatment conditions. Sample sizes on the graphs are numbers of mice. G, Example image showing the extent of Cre-dependent expression of hM3D as indicated by the mCherry reporter expression. H, Example images showing colocalization of mCherry and PV immunolabeling signal in AI neurons. The white arrowhead and pink arrows point to mCherry-only and PV-only cells, respectively. I, Ratio of multiunit spontaneous activity 5 min before and 65 min after CNO injection to activate PV neurons in sham-exposed (n = 32 multiunits/2 mice) and noise-exposed (n = 81 multiunits/3 mice) mice. Noise exposure significantly reduced the inhibitory effect of CNO; *p < 0.05.
