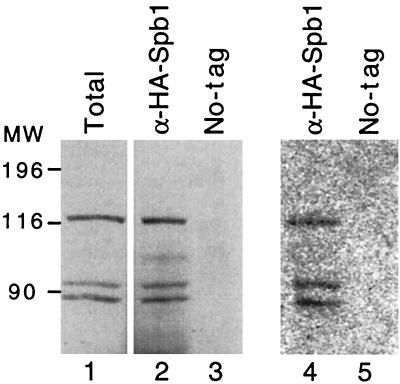
FIG. 12.
Spb1p is radiolabelled after UV cross-linking with [3H]AdoMet. Western blotting with anti-HA antibodies revealed the presence of HA-Spb1p in a total cellular extract prepared from the strain (YDK14-1A/pDK376), along with two degradation products (lane 1). The anti-HA antibodies cross-linked to the protein A-Sepharose beads were used to immunoprecipitate HA-Spb1p from the same extract. The beads were then incubated with [3H]AdoMet before being UV cross-linked for 30 min. Proteins were recovered in a denaturing buffer and analyzed by Western blotting (lane 2). The same experiment was performed with a strain in which Spb1p is not tagged (lane 3). The membrane was exposed for autoradiography for 3 weeks at −70°C with an intensifying screen to reveal the tritiated molecules. Lanes 4 and 5 are identical to lanes 2 and 3. MW, molecular weight markers in thousands.