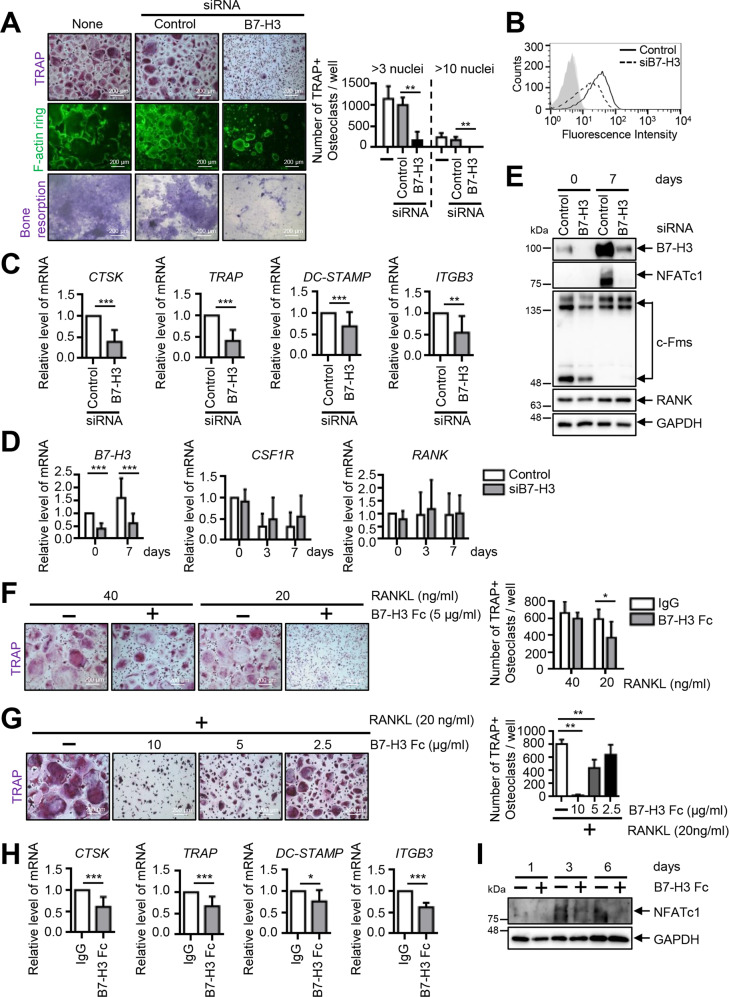
Fig. 2. B7–H3 deficiency suppresses human osteoclast differentiation.
A Prior to transfection, M-CSF (40 ng/ml) was added to monocyte culture for three days. OCPs were transiently transfected with B7–H3-specific siRNA, control siRNA (40 nM), or neither, and then induced to differentiate using M-CSF (40 ng/ml) and RANKL (80 ng/ml) for seven days. After staining for TRAP expression, the TRAP-positive multinucleated cells were counted as osteoclasts (>3 or 10 nuclei). Actin rings in osteoclasts were stained with FITC-phalloidin and bone-resorption pits were stained with 1% toluidine blue O in 0.5% sodium borate (scale bar, 200 μm). B Cell-surface B7–H3 expression was measured using flow cytometry at day 0. The representative histograms are shown (solid line: control siRNA; dashed line: B7-H3-specific siRNA; gray shaded: isotype control). C The expression of mature osteoclast markers, CTSK, TRAP, DC-STAMP, and ITGB3, was analyzed using RT-qPCR at day 7. D The expression of B7–H3, CSF1R, and RANK mRNA during osteoclast differentiation was detected using RT-qPCR. E Whole-cell lysates were immunoblotted with B7–H3, NFATc1, c-Fms and RANK antibodies. F Monocytes were cultured with M-CSF (20 ng/ml) for two days. OCPs were further incubated with M-CSF (20 ng/ml) and RANKL (40 or 20 ng/ml) in the presence of B7–H3–Fc (5 μg/ml) or human IgG for six days. G OCPs were cultured with M-CSF (20 ng/ml) and RANKL (20 ng/ml) in the presence of B7–H3–Fc (10, 5, and 2.5 μg/ml) or human IgG for six days. F, G TRAP staining was performed and the number of TRAP-positive multinucleated cells per well were counted as osteoclasts (scale bar, 200 μm). H OCPs were cultured with M-CSF (20 ng/ml) and RANKL (20 ng/ml) in the presence of B7–H3–Fc (5 μg/ml) or human IgG. The expression of the mature osteoclast markers, CTSK, TRAP, DC-STAMP, and ITGB3, was analyzed at day 8 using RT-qPCR. (I) Whole-cell lysates were immunoblotted with NFATc1 antibody. C, D, H The mRNA levels were normalized relative to GAPDH expression.