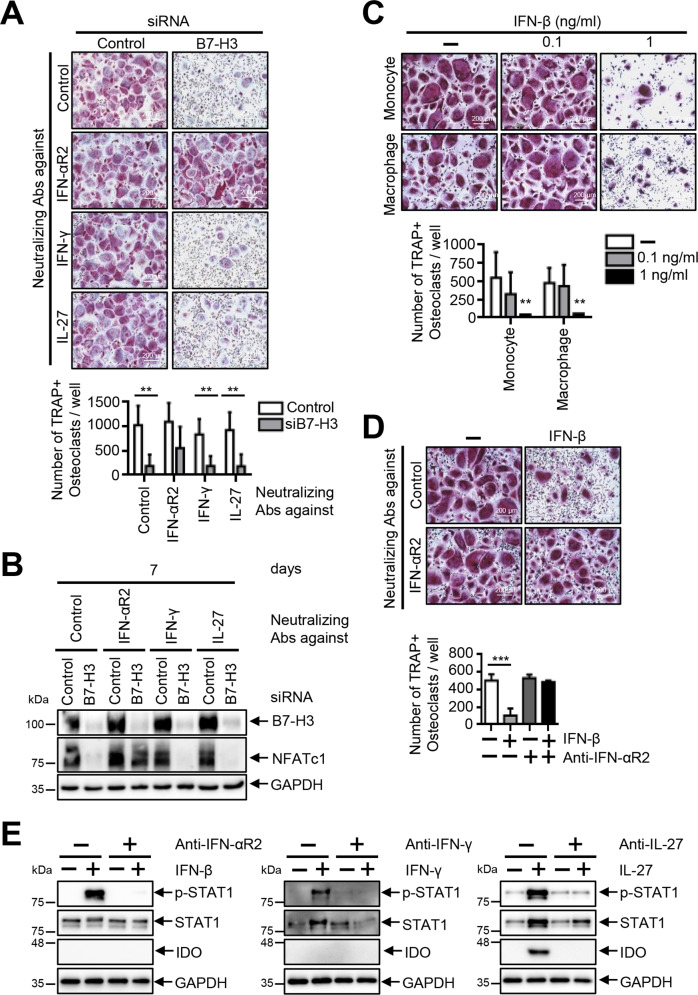
Fig. 4. The inhibition of osteoclastogenesis is restored by the addition of a neutralizing anti-IFN-αR2 antibody in B7–H3-deficient OCPs.
A M-CSF (40 ng/ml) was added in monocyte culture for three days. OCPs were transiently transfected with B7–H3-specific siRNA or control siRNA (20 nM) and then induced to differentiate using M-CSF (40 ng/ml) and RANKL (80 ng/ml) in the presence of neutralizing antibodies for IFN-αR2, IFN-γ, or IL-27 for seven days. B Whole-cell lysates were immunoblotted with B7–H3 and NFATc1 Abs. C Monocytes were cultured with M-CSF (20 ng/ml) in the presence of IFN-β (0.1 and 1 ng/ml) or distilled water for two days and then were further incubated with M-CSF (20 ng/ml) and RANKL (40 ng/ml) in the presence of recombinant IFN-β or distilled water for six days. OCPs, cultured with M-CSF (20 ng/ml) for two days, were induced to differentiate using M-CSF (20 ng/ml) and RANKL (40 ng/ml) in the presence of recombinant IFN-β (0.1 and 1 ng/ml) or distilled water for an additional six days. D OCPs were induced to differentiate using M-CSF (20 ng/ml) and RANKL (40 ng/ml) in the presence of anti-IFN-αR2 and recombinant IFN-β (1 ng/ml) for six days. (A, C, D) TRAP staining was performed and the number of TRAP-positive multinucleated cells per well were counted (scale bar, 200 μm). E Monocytes were incubated with or without neutralizing antibodies (2 μg/ml) against IFN-αR2, IFN-γ, or IL-27 for 1 h. Control antibodies were used at the equal concentration. At the end of the duration of culture, the cells were treated with recombinant IFN-β (10 ng/ml) or IFN-γ (10 ng/ml) for 10 m or recombinant IL-27 (50 ng/ml) for one day. The expression of phospho-STAT1 (PY701), STAT1, and IDO protein was evaluated via Western blot.