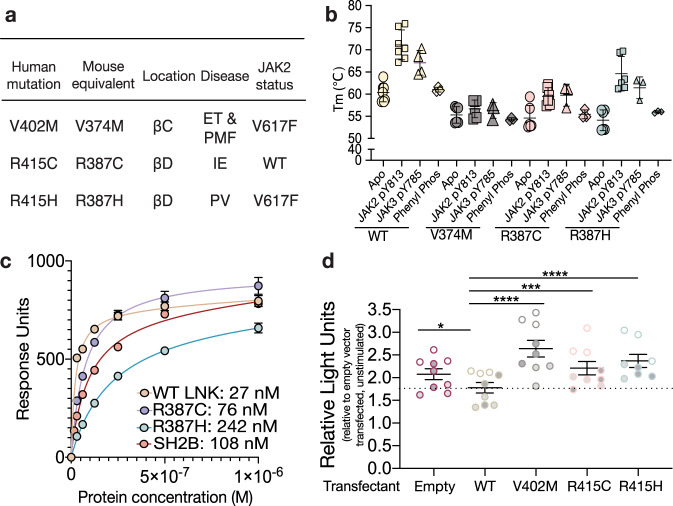
Fig. 5. Characterisation of three LNK SH2 domain mutations.
a Three variants investigated in this study, their location within the LNK SH2 domain, the disease they were identified in (PV polycythemia vera, ET essential thrombocythemia, PMF primary myelofibrosis, IE idiopathic erythrocytosis) and the JAK2 status of the patient. b Melting temperature (Tm) in °C for apo LNK SH2 domains and with JAK2 pY813 and JAK3 pY785 peptides and the pTyr mimetic, phenyl phosphate (Phenyl phos). Data are displayed as the mean ± SD of technical replicates from n = 3 independent experiments. c Fitted curves for WT, R387C and R387H LNK SH2 domain, and SH2B SH2 domain affinity for JAK2 pY813. Data are displayed as mean ± SD from n = 5 independent experiments for WT and R387C LNK SH2 domains and n = 4 independent experiments for LNK R387H and n = 3 independent experiments for SH2B SH2 domains. KD for each SH2 domain is indicated in nM. V374M did not bind. d Relative GAS-Firefly luciferase activity after co-transfection of HEK293 with WT, V402M (V374M), R415C (R387C) or R415H (R387H) full-length human LNK mutants and treatment with 50ng/ml rhIFN-γ. Data are displayed as mean ± SEM from n = 3 independent experiments where data were pooled from three experiments with each data point representing the mean of triplicate replicates of a single transfection normalised to the mean RLU (relative light units) of unstimulated empty vector control in each experiment. Statistical analysis was performed using two-sided pairwise multiple comparison with Tukey’s adjustment under estimated marginal means (emmeans) function based on linear mixed-effect models using each individual experiment as a block. Significance is indicated with asterisks: *p < 0.05, ***p < 0.001, ****p < 0.0001 (empty control p = 0.05, V402M p < 0.0001, R415C p = 0.0006 and R415H p < 0.001).