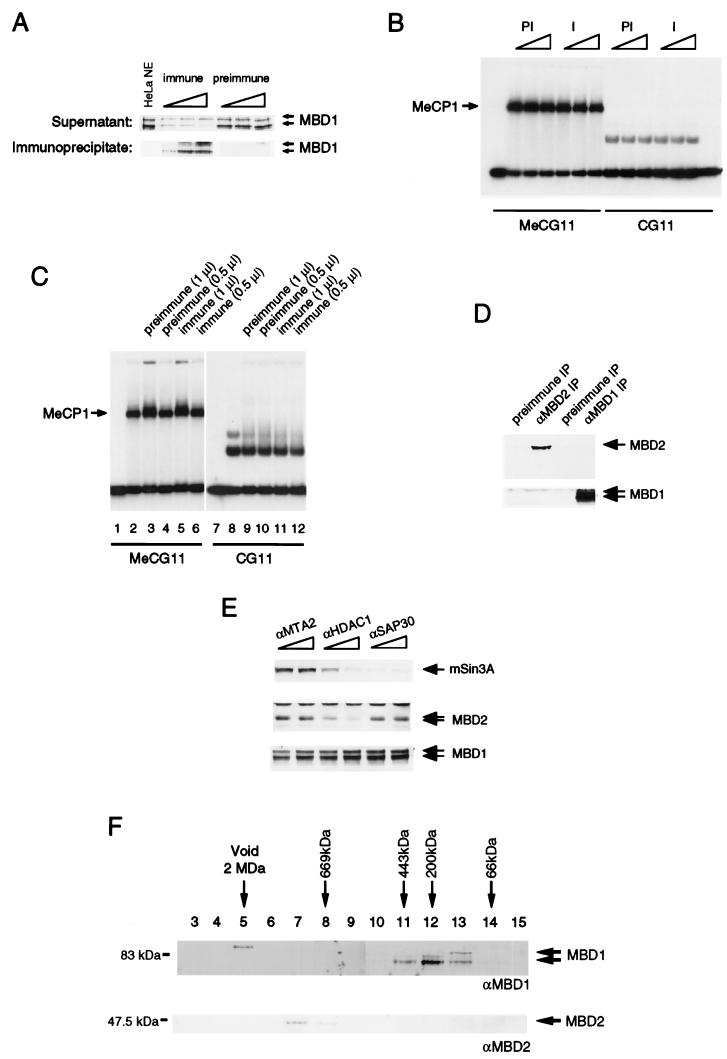
FIG. 3.
MBD1 is not a component of HeLa MeCP1 activity. (A) Extracts subjected to adsorption by anti-MBD1 antibodies (immune) contained reduced levels of MBD1, whereas preimmune antibodies did not deplete MBD1. Only immunoprecipitates by anti-MBD1 antibodies contained MBD1. We used 1, 5, and 10 μl of sera for the experiments. (B) Both MBD1-immunodepleted extracts (I) and mock-depleted extracts (PI) retained MeCP1 activity, as assayed by the formation of a complex with methylated probe DNA. MeCG11 is the methylated version of CG11. (C) Antibody against MBD1 did not supershift MeCP1 activity. Serum was not added in lanes 2 and 8. Lanes 3, 5, 9, and 11 contain 1 μl of preimmune or immune serum. Half the amount of serum was used in lanes 4, 6, 10, and 12. The complexes formed with the nonmethylated probe are nonspecific complexes not due to MeCP1 (7). (D) MBD1 and MBD2 are not associated in the same complex. Anti-MBD1 antibodies did not immunoprecipitate MBD2, and anti-MBD2 antibodies did not immunoprecipitate MBD1. (E) MBD1 is not associated with the MBD2-HDAC1 MeCP1 complex. Anti-HDAC1 antibodies immunodeplete MBD2 but not MBD1. Anti-MTA2 and anti-SAP30 antibodies were used as controls, since neither MBD1 nor MBD2 associates with these proteins. (F) Apparent molecular masses of MBD1 and MBD2. HeLa nuclear extract was applied to a Superose 6 gel filtration column. Eluted fractions were analyzed by Western blotting to detect MBD1 and MBD2.