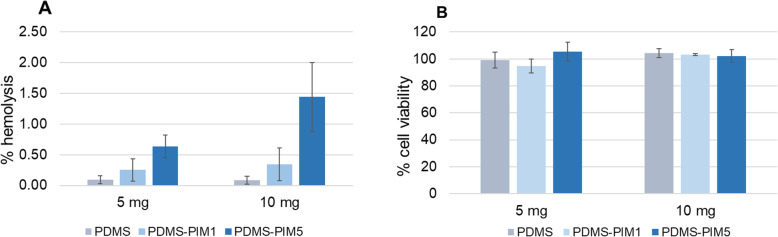
Fig. 7.
Biocompatibility analysis. (A) Hemocompatibility assessment was carried out using red blood cell hemolysis assay. PDMS-PIM biomaterial (5 mg and 10 mg) in 100 μl of red blood cells was incubated at 35 °C at 150 rpm for 1 h. The lysis of RBC was determined spectrometrically at 576 nm. (B) Cytocompatibility evaluation was carried out using L929 fibroblast cell viability assay. PDMS-PIM biomaterial (5 mg and 10 mg) was added to the L929 cells (in 96 well plate) in 100 μl of DMEM complete media. The plate was incubated at 35 °C with 5% CO2 for 24 h. Cell viability was determined using the Alamar Blue reagent. The results are expressed as mean and standard deviation of three replicates