Figure 1.
AA/FBS induces FKBP12 acetylation and involves in regulating cellular viral infection activity
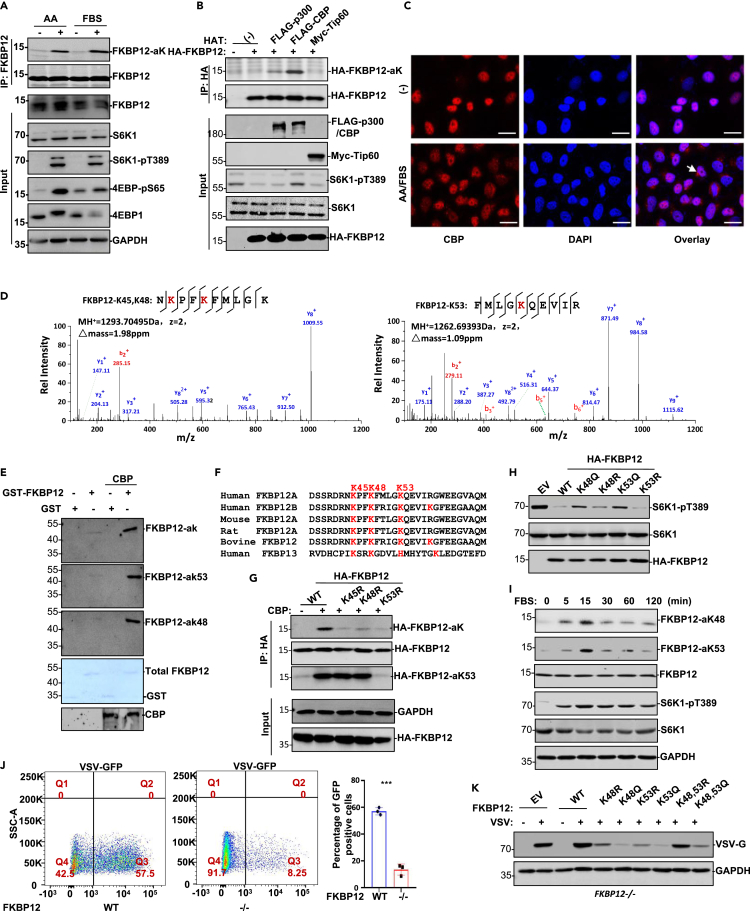
(A) Mouse fibroblasts starved for serum/AA for 24 h followed by AA or FBS treatment (30 min). FKBP12 immunoprecipitates prepared from WCL of these fibroblasts were analyzed in Western blot with pan anti-acetyl-K.
(B) In HEK293T cells, HA-FKBP12 was cotransfected with p300, CBP or Tip60. FKBP12 immunoprecipitates were analyzed in Western blot with pan anti-acetyl-K.
(C) Immunofluorescence staining of CBP with anti-CBP. DAPI was used for nuclear counter staining. Scale bar: 20 μm.
(D) Acetyl-FKBP12 proteins were purified from HEK293T cells with Flag-FKBP12 and HA-CBP cotransfection. Mass spectrometry analysis of trypsinized acetyl-FKBP12 proteins uncovered K45, K48, and K53 as the acetyl-residues.
(E) GST-FKBP12 recombinant protein was acetylated by CBP in vitro.
(F) Alignment of the K-cluster of FKBP12 from different species.
(G) FKBP12-K45R, -K48R, and -K53R variants were compared with wild type FKBP12 for acetylation induction by CBP in HEK293T cells with HA-FKBP12 transfected along with CBP.
(H) HA-FKBP12 WT and its mutants were transiently transfected into 293T cells, WCLs were determined with the indicated antibodies.
(I) As indicated, a time course to reveal FBS induced FKBP12 acetylation and S6K1 phosphorylation induction in FBS/AA starved mouse fibroblasts. Polyclonal antibodies against FKBP12-aK48 and FKBP12-aK53 were constructed for FKBP12 acetylation analysis. (J) WT or FKBP12−/− mouse fibroblasts infected with VSV, the infected cells were analyzed by flow cytometry. Left, representative image; right, statistical results of 3 independent experiments. Data are represented as mean ± SD; ∗∗∗, p < 0.001, two-tailed t test. (K) In FKBP12−/− mouse fibroblasts, indicated FKBP12 KR or KQ variants were overexpressed followed by VSV infection. VSV-G protein was analyzed in Western blot.