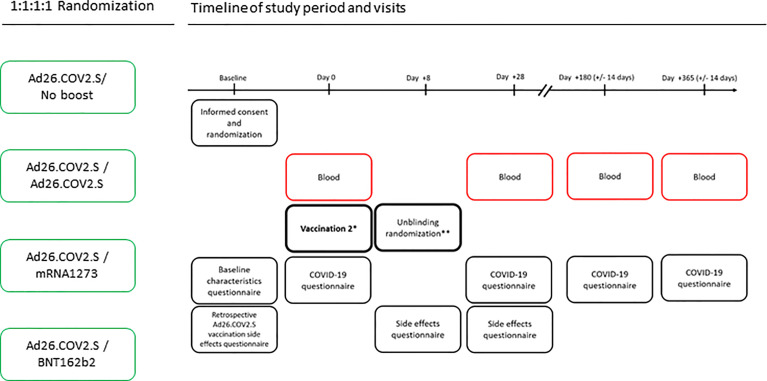
Figure 1.
SWITCH trial design. The SWITCH trial includes four arms that receive a booster vaccination 84 days after prime with Ad26.COV2.S. Blood samples will be collected at indicated timepoints by venepunctures. Questionnaires will be performed to collect reactogenicity data and to determine whether participants had breakthrough infections despite vaccinations. *One of the four arms or 25% of the randomized participants will not receive a second vaccination; **Unblinding the randomization of participants will be done after the side effects (i.e., reactogenicity) questionnaire is completed.