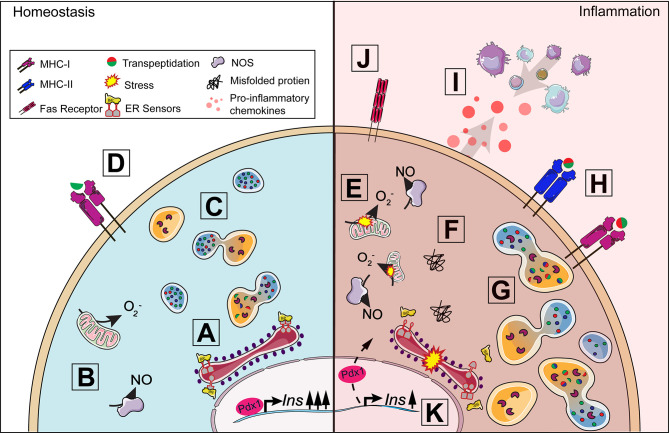
Figure 2.
Beta-cell response to inflammation. Under homeostasis conditions, insulin production is tightly coupled with cellular metabolism including protein synthesis in the endoplasmic reticulum (ER) (A) and mitochondrial function (B). When insulin secretory granule proteins are in excess, they can be broken down and recycled by crinophagy, a process by which granules fuse with lysosomes (C). Some peptides from this degradation process are presented on MHC-I (D) and, in healthy cells, should not lead to activation. A proinflammatory environment around the islet exacerbates ER and oxidative stress (E) contributing to the dysregulation of multiple processes in the beta-cell. The accumulation of misfolded proteins can result in the activation of the unfolded protein response (F) and increase lysosomal degradation of insulin secretory granule proteins (G). Protein degradation under stress can lead to the production of neo-antigens, such as hybrid insulin peptides, through transpeptidation. ER and oxidative stress results in the upregulation of MHC-I and the unique expression of MHC-II (H) by the beta-cell allowing for increased presentation of potential neo-antigens to T cells. Fas receptor expression (J) makes the beta-cell vulnerable to Fas-mediated apoptosis. The release of chemokines (I) from the beta-cell further contributes to immune cell recruitment and the development of insulitis. Insulin production can be affected as disturbances in cellular homeostasis can lead to the translocation of Pdx1 from the nucleus to the cytoplasm, decreasing insulin production (K).