Abstract
Endocrine disrupting chemicals (EDCs) are chemicals found in our environment that interrupt typical endocrine function. Some flame retardants (FRs) are EDCs as shown in their interaction with steroid and nuclear receptors. Humans are consistently exposed to flame retardants as they are used in everyday items such as plastics, clothing, toys, and electronics. Polybrominated diphenyl ethers were used as the major FR until 2004, when they were replaced by organophosphate flame retardants (OPFRs). Previous research in rodent models utilizing a commercial flame retardant mixture containing OPFRs reported alterations in anxiety-like behavior in the elevated plus maze (EPM) for rodents perinatally exposed to OPFRs. In the present study we utilize wild-type mice maternally exposed (gestational day 7 to postnatal day 14) to either an OPFR mixture of tris(1,3-dichloro-2-propyl), triphenyl phosphate, and tricresyl phosphate or a sesame seed oil vehicle. These mice were evaluated for anxiety-like behavior in adulthood on the open field test (OFT) and the light/dark box (LDB) as well as the EPM. Outcomes from the OFT and LDB indicate that males and females maternally exposed to OPFRs exhibit altered locomotor activity. Results of the EPM were sex-specific as we did not observe an effect in females; however, effects in males differed depending on exposure condition. Males maternally exposed to OPFRs exhibited an anxiolytic-like phenotype in contrast to their vehicle counterparts. This effect in perinatally OPFR-exposed males was not due to alterations in locomotor activity. Our research illustrates that there are sex- and exposure-dependent effects of OPFR exposure on locomotor and anxiety-like behaviors in a mouse model.
Keywords: Endocrine disruptors, Organophosphate flame retardants, Anxiety, Maternal exposure, Sex differences
1. Introduction
Endocrine disrupting chemicals (EDCs) are a class of chemicals that interfere with the endocrine system and cause adverse effects on an organism (Bergman et al., 2013; Zoeller et al., 2012). In humans and in animal models, disruption of the endocrine system produces serious developmental, reproductive, neurological, and immune malfunctions (Dickerson and Gore, 2007; Kajta and Wójtowicz, 2013; Rogers et al., 2013). Industry and manufacturing have exponentially increased EDC contaminants in our environment, and these toxic chemicals have been used with unforeseen consequences (Giesy et al., 2002; Lintelmann et al., 2003). In concert with environmental exposure, humans are in contact with EDCs daily as they are found in common consumer products such as plastic bottles, pesticides, and flame retardants (FRs) (Wong and Durrani, 2017). Unfortunately, this proliferation of EDCs in our environment is now considered a major public health concern.
FRs are a primary source of EDC exposure. FRs are chemical additives in materials that reduce flammability by delaying combustion (Alaee et al., 2003). Accordingly, many products with FR additives are found in a residential setting, as they are thought to provide occupants additional time to escape from fires (Stapleton et al., 2009). The constituents of FRs are not completely bound to these materials and leach out over time, accumulating in household dust where they are inadvertently ingested by humans (Kim et al., 2006). Because children display frequent hand-to-mouth or object-to-mouth contact they are at particular risk for FR exposure (Hoffman et al., 2015; Stapleton et al., 2008; Stapleton et al., 2014; Xue et al., 2007). FRs such as tris(1,3-dichloro-2-propyl) phosphate (TDCPP) have been detected in household dust (~2000 ng/g) (Dodson et al., 2012). FRs have been found in the urine of children. Bis(1,3-dichloroisopropyl) phosphate (BDCIPP), a metabolite of TDCPP, was observed at a median concentration of ~2.8 ng/ml (Butt et al., 2016). Similar observations are reported worldwide. For example, BDCIPP was present in 100% of samples at a median concentration of 7.8 μg/g in the urine of Australian children (He et al., 2018). Moreover, considerable levels of FRs have been detected in human blood (2,2’,4,4’,5,5’-hexabromodiphenyl ether, median concentration 0.13−3.1 pmol/g) (Sjödin et al., 2001) and breast milk (tris-2-butoxyethyl phosphate, mean concentration 1.44 ± 0.789 ng/mL) (Ma et al., 2019). FRs are even found in the workplace; for example, low μg/g TDCPP concentrations have been observed in office dust (mean 6.06 μg/g) (Carignan et al., 2013).
Polybrominated diethyl ethers (PBDEs) were the prominent FR for commercial use from the 1960s to the early 2000s, when several adverse health effects of PBDEs were identified. PBDEs have since been phased out by way of regulatory or voluntary measures (Daub, 2004). The phasing out of PBDEs triggered a need for alternatives, leading to an increase in organophosphate flame retardant (OPFR) usage to meet safety standards (Dodson et al., 2012). OPFRs are comprised of several compounds, including chlorinated alkyl phosphates and non-halogenated aryl phosphates. Chemical formulations include triphenyl phosphate (TPP), found in the well-known FR mixture Firemaster® 550 (FM 550), along with TDCPP and tricresyl phosphate (TCP). It is thought that OPFRs interact with nuclear receptors fundamental to neuroendocrine function and energy homeostasis, such as estrogen receptor (ER)α and peroxisome proliferator-activated receptor (PPAR)γ (Belcher et al., 2014; Liu et al., 2013; Liu et al., 2012). OPFRs are now the most common FRs detected in consumer products (Cooper et al., 2016; Stapleton et al., 2011).
Maternal exposure to OPFRs especially during sensitive periods of development may pose considerable harm to the emerging fetus. Recent work suggests that OPFRs cross the placenta in rats to influence the developing fetus (Baldwin et al., 2017; Phillips et al., 2016). Thus, it is likely that maternal OPFR exposure may lead to neurodevelopmental reprogramming, subsequently inducing long-term behavioral changes in the adult offspring. One particular behavioral phenotype that may be influenced is anxiety-like behavior. In human studies, perinatal EDC exposure is associated with increased anxious states in children (Perera et al., 2016; Perera et al., 2012; Roen et al., 2015), and work in perinatal EDC-exposed animal models does yield alterations in anxiety-like phenotypes later in life (Matsuda et al., 2012; Patisaul and Bateman, 2008; Ryan and Vandenbergh, 2006; Tian et al., 2010; Xu et al., 2012). Moreover, recent work in adult rats suggests perinatal exposure to the FR mixture FM 550 disrupts anxiety-like behavior in a sex-specific manner (Baldwin et al., 2017; Patisaul et al., 2013).
The effects of maternal exposure to OPFRs on anxiety-like behavior have not been well-documented. Here we utilized mice maternally exposed (gestational day 7 to postnatal day 14) to either an OPFR mixture of TDCPP, TPP, and TCP or a sesame seed oil vehicle. These mice were evaluated in adulthood on tests that measure anxiety-like behavior: the open field test (OFT), the light/dark box (LDB) and the elevated plus maze (EPM). Our study aimed to evaluate OPFR maternally exposed male and female adult mice to determine if there are alterations in anxiety-like behavior or locomotor activity.
2. Materials and methods
2.1. Animals and housing conditions
Adult (> 60 days old) wild-type C57/BL6J male and female virgin mice were bred in-house, housed in standard laboratory cages with food (LabDiet PicoLab Verified 5v75 IF, < 75 ppm phytoestrogens; Lab Diet, St. Louis, MO, USA) and water available ad libitum. Mice were maintained under a 12:12 light/dark cycle (lights on at 0800 h) and kept under controlled temperature (21–23°C) and humidity (30–70%) regulation. Breeding pairs were established by pair or trio housing. All animal procedures were completed in compliance with institutional guidelines based on National Institutes of Health standards and were performed with Institutional Animal Care and Use Committee approval at Rutgers University.
2.2. Chemicals
The OPFR mixture consisted of tris(1,3-dichloro-2-propyl)phosphate (TDCPP, Sigma Aldrich, St. Louis, MO, CAS#: 13674–87-8, 95.6%), triphenyl phosphate (TPP, Sigma Aldrich, St. Louis, MO, CAS#: 115–86-6, 99%), and tricresyl phosphate (TCP, AccuStandard, New Haven, CT, CAS#: 1330–78-5, 99%). As previously reported (Krumm et al., 2017), to prepare the OPFR stock, 100 mg of each compound was dissolved into 1 ml of acetone. To prepare the OPFR working stock, 100 μl of OPFR stock was dissolved into 10 mL of sesame seed oil and allowed to vent on a stir plate for 24–48 h, as previously reported (Krumm et al., 2017). Dams were orally administered 1 mg/kg/day of the OPFR mixture or sesame seed oil vehicle, as this dose was previously found to be effective in mouse (Krumm et al., 2017) and rat models (Baldwin et al., 2017; Patisaul et al., 2013). The OPFR mixture or sesame seed oil vehicle was dissolved fresh daily in dehydrated peanut butter.
2.3. Maternal exposures
The baseline weights of females were taken and recorded throughout pregnancy. Gestational day (GD) 0 was determined with confirmation of a vaginal plug. Upon confirmation of sufficient weight gain of pregnancy, on GD 3 all mice were acclimated to an oral dose of a peanut butter–sesame seed oil mixture (30 μl sesame oil in dehydrated peanut butter). On GD 7, males were removed and dams were individually housed. Dams were randomly assigned to be orally dosed with the OPFR mixture or vehicle from GD 7 to postnatal day (PND) 14. This exposure window was used in order to reduce any potential effect on implantation and embryogenesis, and to include the hormone-sensitive developmental time period from birth to the end of lactation. Daily ingestion of the OPFR mixture or vehicle occurred at 1000 h. Dams were individually housed during daily dosing period, and afterward returned to their home cage. Dams were also individually housed during lactation. Pups were weaned at PND 21 and housed by sex within litter (2–4 per cage).
2.4. Behavioral testing
At 8 weeks, all remaining offspring were handled for 1 min/day for five days before task onset. Between weeks 8 and 9, mice were subjected to behavioral testing consisting of the OFT, EPM, and LDB. Behavioral testing was conducted between 0900–1200 h and occurred during the light phase of the light:dark cycle. Testing during a particular estrous cycle stage was not controlled. Behavior was recorded (ANY-maze, Version 6, Stoelting, USA) by a camera suspended above the test arena. All mice were habituated to room conditions for 24 h before testing. All tests were conducted by an experimenter blind to the exposure group or sex.
2.4.1. Open field test
The OFT evaluates anxiety-like behavior in concert with spontaneous exploratory activity (Gould et al., 2009). Mice were first subjected to a 10 min OFT, which consisted of a square white opaque arena area (40 cm long × 40 cm wide × 40 cm high, open-top) with a 64-square grid floor (8 × 8 squares, 5 cm/side). Mice were placed in the same (10 cm long × 10 cm wide) corner square of the arena and were allowed to freely explore for 10 min. Automated ANY-maze computer-scored measures consisted of the amount of time the animal spent in the 10 cm center zone (4 × 4 center squares), corners (4 × 4 corner squares), and perimeter (48 perimeter squares). All four paws of the animal’s body needed to be in a zone to be defined as an entry. Furthermore, distance traveled (m) throughout the entire apparatus was evaluated. After each animal trial, the surface of the arena was cleaned with a sterilizing solution (LabSans). Upon completion of testing, the animal was returned to its home cage overnight.
2.4.2. Elevated plus maze
Mice were next assessed on the EPM. The EPM assesses anxiety-like behavior and is often used in combination with the OFT (Walf and Frye, 2007). The apparatus consisted of two sets of opposing arms (30 cm × 5 cm) extending from a central (5 cm × 5 cm) region. Two arms were enclosed (15-cm-high walls) and two arms remained open. Animals were placed in the central area of the maze in the direction of a closed arm and allowed to freely explore both open and closed arms for 5 min. All four paws of the animal’s body needed to be in a zone to be defined as an entry. ANY-maze computer-scored measures included the following: the number of open arm entries, time spent in open arms, time spent at the end of the open arms, percentage of time in the open arms [open arm time/(open + closed arm time)], percentage of entries in the open arms [open arm entries/(open + closed arm entries)], and total arm entries (open + closed arm entries). Following each animal trial, the surface of the arena was cleaned with LabSans and the animal was returned to its home cage overnight.
2.4.3. Light/dark box
The LDB is another task that evaluates anxiety-like behavior and spontaneous exploratory activity through a natural conflict situation between innate exploratory desire of novelty and tendency to avoid the unfamiliar (Bourin and Hascoët, 2003). The LDB assessment took place using a square white opaque arena (40 cm long × 40 cm wide × 40 cm high, open-top), with a black rectangle insert (40 cm long × 20 cm wide × 40 cm high, closed top) that contained an opening (7.5 cm × 7.5 cm) located at floor level in the center of the partition. Each mouse was placed in the same corner of the light side of the apparatus and allowed to freely explore for 10 min. All four paws of the animal’s body needed to be in a zone to be defined as an entry. ANY-maze computer-scored measures consisted of the time in the light zone, time in the transition zone (10 cm × 5 cm, in front of dark zone entrance), distance traveled (m), and speed (m/sec). After each animal trial, the surface of the arena was cleaned with LabSans and the animals were returned to its home cage.
2.5. Statistical analysis
All behavioral data were analyzed with a 2 × 2 ANOVA with exposure (oil × OPFR) and sex (male × female) as factors on Prism 6 (GraphPad Software, La Jolla, CA, USA). Planned comparisons were analyzed within each sex through Student’s t-tests. A total of 34 dams were dosed: 19 dams were dosed with vehicle and 15 dams were dosed with the OPFR mixture. Three dams did not get pregnant, and 3 dams (2 vehicle and 1 OPFR) gave birth to less than 6 pups and were excluded. On PND 0 and PND 14, 1 male and 1 female pup from each litter were randomly selected to be sacrificed in designation for another project. Between 1 and 6 offspring were used per litter, and we used the average for each litter within each sex as one datapoint. There were a total of four groups: oil:male, n = 9; oil:female, n = 6; OPFR:male, n = 6; OPFR:female, n = 7. Values from individual mice that exceeded 2 standard deviations (SD) above or below the group mean were considered outliers and excluded. The results are reported as mean (± SEM) and results were considered statistically significant at p < .05. Effect sizes are presented as eta squared (η2) for two-way ANOVAs and Cohen’s d (d) for t-tests (Cohen, 1988; Cohen, 2013). For each ANOVA, η2 effect sizes were determined by dividing the sum of squares of an effect by the total sums of squares for all effects, interactions, and error (Cohen, 1973). The d effect sizes were calculated as the difference between the mean of the two groups, divided by the SD (Cohen, 1988; Cohen, 2013; Sullivan and Feinn, 2012). Effect size guidelines for η2 are 0.01 = small, 0.05 = medium, and 0.14 = large; effect size guidelines for d are 0.2 = small, 0.5 = medium, and 0.8 = large effect sizes (Cohen, 1973; Cohen, 1988; Sullivan and Feinn, 2012).
Statistical outliers for each measure are as follows. Outliers for the OFT: Outliers for perimeter time, n = 1, oil:male; number of corner entries, n = 1, oil:male; time spent (sec) in the 10 cm center zone, n = 0; distance traveled, n = 0. Outliers for the EPM: number of open-arm entries, n = 0; time spent in the open arms (sec), n = 1, OPFR:female; open-arm end time (sec), n = 1, OPFR:female, percentage of open-arm entries, n = 1, OPFR:female; percentage of time spent in the open arms, n = 1, oil:male; number of total arm entries, n = 0. Outliers for the LDB: time spent in the light zone (sec), n = 1, oil:male; time spent in the transition zone (sec), n = 0; total distance traveled (m), n = 0; speed (m/sec), n = 0.
3. Results
3.1. Open field test
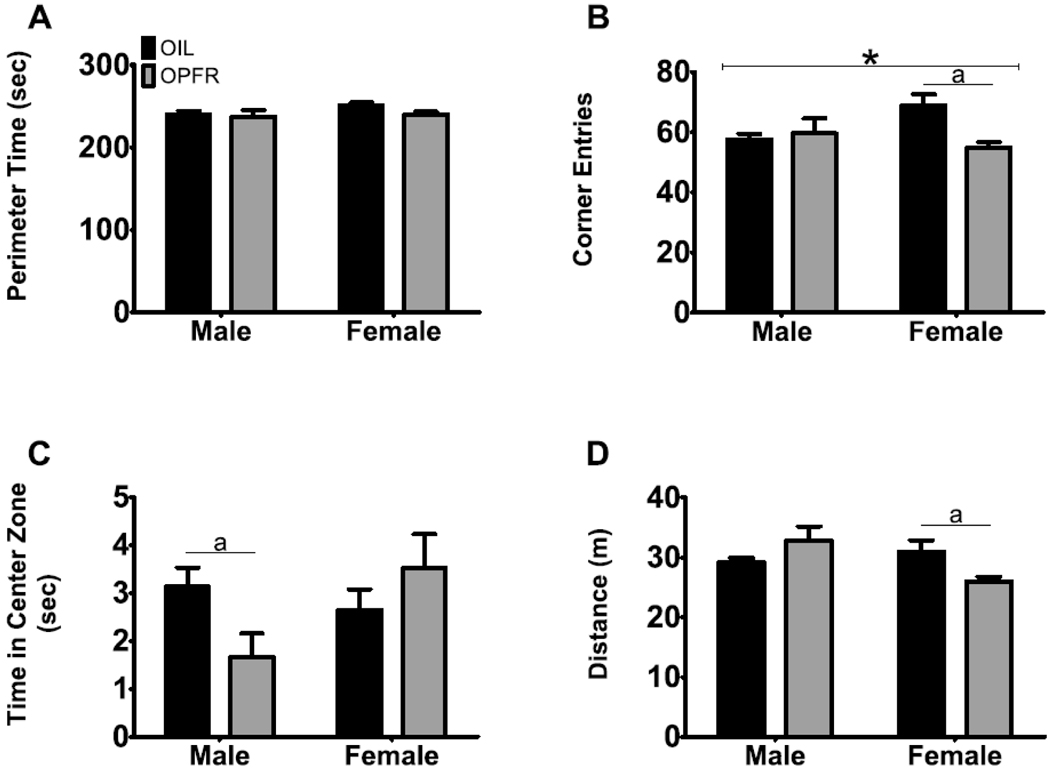
The OFT was utilized to assess exploratory and anxiety-like behavior. The evaluation of perimeter time (Fig. 1A) revealed no effect of exposure, sex, or interaction. On number of corner entries (Fig. 1B), we did not observe an effect of sex; however, we did observe an effect of exposure (F(1,23) = 4.19, p = .045, η2 = .002) and an interaction between sex and exposure (F(1,23) = 7.30, p = .0091, η2 = .003). Subsequent analysis revealed that OPFR-exposed females exhibited a decrease in the number of corner entries on the OFT (p = .0015, d = 1.36); however, there was no observable effect in OPFR males on this measure. Next, we evaluated the time spent (sec) in the 10-cm center zone (Fig. 1C). Although we did not detect a main effect of exposure or sex, an interaction between exposure and sex was found (F(1,24) = 4.57, p = .037, η2 = .025). Follow-up analysis identified that males maternally exposed to OPFRs had a significant reduction in time spent (sec) in the 10-cm center zone (p = .042, d = .84). No differences were observed in 10-cm center zone time (sec) in OPFR-exposed females. Overall locomotor activity was measured (Fig. 1D) because differences in locomotion may confound behavioral assessments of emotionality (Archer, 1973; Gould et al., 2009). Results indicated no effect of exposure, a trending effect of sex (F(1,24) = 3.47, p = .068), and an interaction of exposure and sex (F(1,24) = 9.73, p = .0029, η2 = .004). Maternally exposed OPFR females showed a significant decrease in total distance traveled (m) on the OFT (p = .023, d = .949). OPFR-exposed males exhibited an inverse trend on total distance traveled (m) (p = .061). Results suggest that males and females maternally exposed to OPFRs are differentially affected in locomotor activity on this task.
Fig. 1. Males and females maternally exposed to OPFRs differed on locomotor behavior in the open field test (OFT).
A) No effects were observed in perimeter time (sec). B) OPFR-exposed females exhibited a decrease in the number of entries in the corners of the OFT, in contrast to same-sex oil controls. We saw no effect in OPFR-exposed males in the number of corner entries. C) Maternal exposure to OPFRs reduced time in the 10-cm center zone in males; however, no effect was detected in females on this measure. D) OPFR exposure decreased distance traveled (m) in females. OPFR-exposed males exhibited an inverse trend. This suggests that males and females maternally exposed to OPFRs are differentially affected in locomotor activity on this task. */a = p < .05; capped lines = exposure effect; a = pairwise difference between exposure within sex; data are represented as mean ± SEM.
3.2. Elevated plus maze
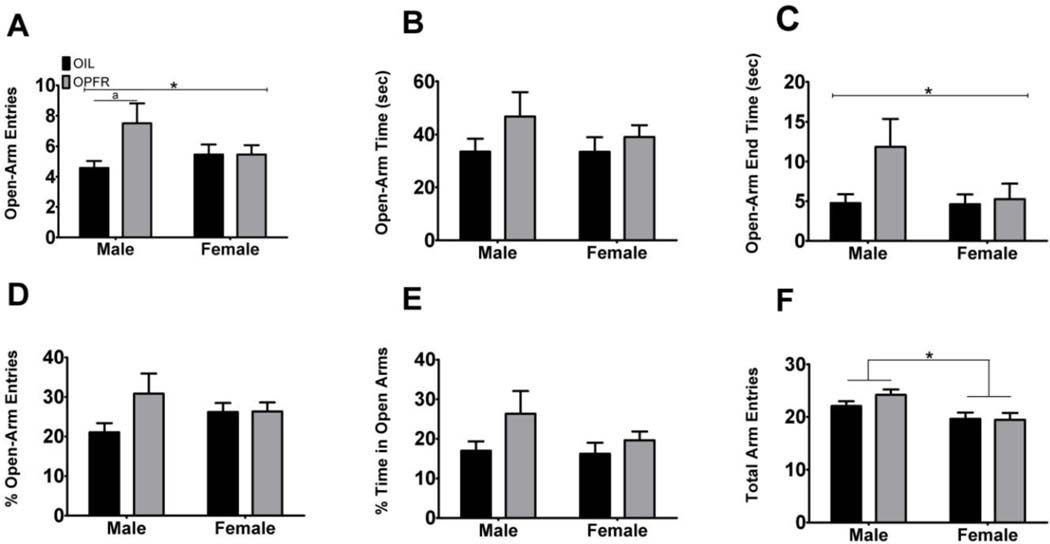
The EPM was also used as a measure of anxiety-like behavior in maternally exposed OPFR males and females. The assessment of open-arm entries (Fig. 2A) did not result in an effect of sex; however, an effect of exposure (F(1,24) = 4.20, p = .045, η2 = .013) and an interaction effect (F(1,24) = 4.17, p = .046, η2 = .013) were observed. Subsequent analysis revealed that OPFR-exposed males exhibited a significant increase in the number of open-arm entries in contrast to same-sex oil controls (p = .014, d = 1.00). We did not detect an effect in females on the number of open-arm entries. Next, no effect of exposure, sex, or interaction was observed in open-arm time (sec) (Fig. 2B). The analysis of open arm end time (Fig. 2C) revealed an effect of exposure (F(1,23) = 4.24, p = .044, η2 = .039) and a trend for both sex (F(1,23) = 3.24, p = .077) and interaction (F(1,23) = 2.94, p = .092). There were no significant effects of exposure, sex, or interaction on percentage of open-arm entries (Fig. 2D), although there was a trend of exposure (F(1,23) = 2.85, p = .097). Furthermore, percentage of time in the open arms (Fig. 2E) identified a trend of exposure (F(1,23) = 4.00, p = .051), yet no effect of sex or interaction. Lastly, in the assessment of locomotor activity on the EPM, we evaluated total arm entries (Fig. 2F). We did not identify effects of exposure or interaction, although we did observe an effect of sex (F(1,24) = 9.40, p = .0033, η2 = .006). Together, these results indicate that the anxiolytic-like behavior observed in OPFR-exposed males is not due to differences in locomotor activity.
Fig. 2. Maternally exposed OPFR males exhibit anxiolytic-like behavior on the elevated plus maze (EPM).
A) Maternal exposure to OPFRs increased the number of open-arm entries in males; however, no effect was observed in females on this measure. B) No effects were detected in open-arm time (sec). C) A main effect of exposure was observed in open-arm end time (sec). D) No effects were observed in percentage of open-arm entries. E) No effects were observed in percentage of time in open arms. F) A main effect of sex in total arm entries was observed, such that regardless of exposure males had a greater amount of arm entries than females. These results suggest that the anxiolytic-like behavior observed in OPFR-exposed males compared to same-sex oil controls is not due to exposure differences in locomotor activity. */a = p < .05; capped lines = exposure effect; bracketed lines = sex effect; a = pairwise difference between exposure within sex; data are represented as mean ± SEM.
3.3. Light/dark box
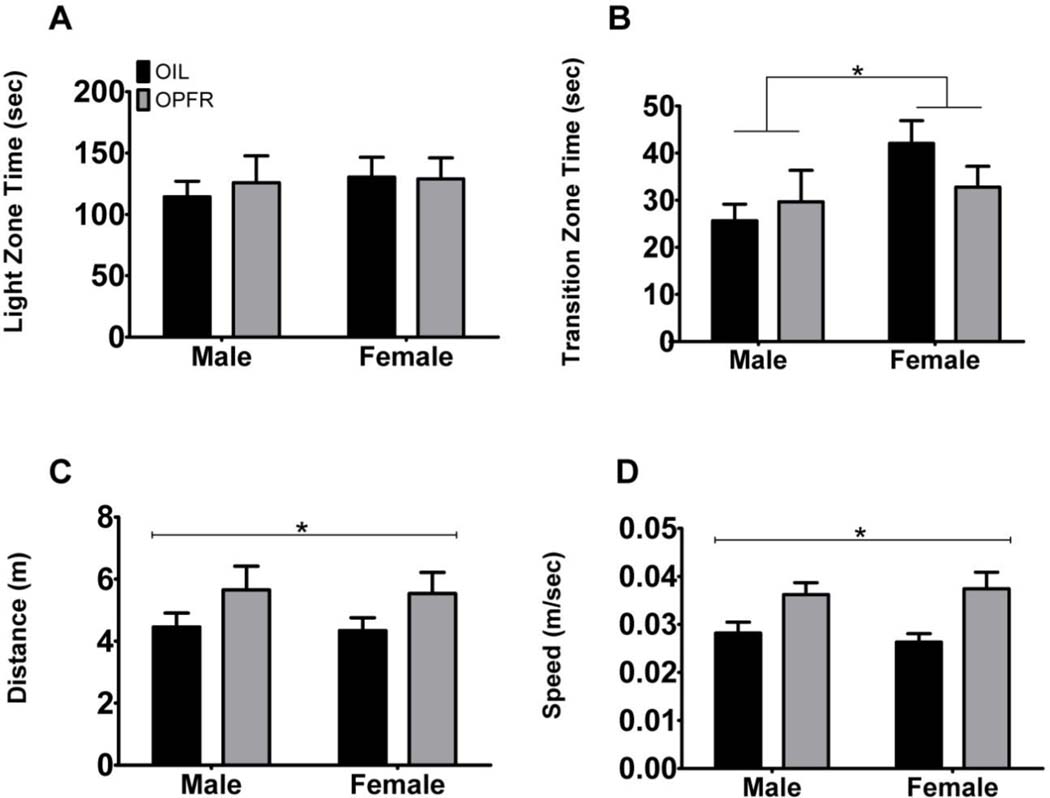
We utilized the LDB in conjunction with the OFT and EPM to assess exploratory and anxiety-like behavior. In the assessment of time spent in the light zone (sec) of the LDB (Fig. 3A), we did not observe an effect of exposure, sex, or interaction. The evaluation of time spent in the transition zone (sec) (Fig. 3B) yielded no effect of exposure or interaction; however, we did identify an effect of sex (F(1,24) = 5.25, p = .026, η2 = .020). Next, we assessed total distance traveled (m) (Fig. 3C) and detected an effect of exposure (F(1,24) = 6.04, p = .017, η2 = .016), but no sex or interaction effect. Lastly, the evaluation of speed (m/sec) (Fig. 2D) identified an effect of exposure (F(1,24) = 12.56, p = .00085, η2 = .016), but no effect of sex nor interaction. Collectively, these outcomes suggest that maternal exposure to OPFRs alters locomotor behavior in male and female adult offspring in this task.
Fig. 3. Males and females maternally exposed to OPFRs displayed increased locomotor activity in the light/dark box (LDB).
A) No effects were observed in time spent in the light zone (sec). B) Females had an increase in time spent in the transition zone (sec) regardless of exposure. C) OPFR exposure increased the total distance traveled (m) in males and females when compared to their same-sex oil controls. D) Maternal exposure to OPFRs increased speed (m/sec) in both males and females. These results suggest OPFR exposure in both males and females alters locomotor behavior in this task. */a = p < .05; capped lines = exposure effect; bracketed lines = sex effect; data are represented as mean ± SEM.
4. Discussion
Maternal exposure to OPFR during the perinatal period altered adult non reproductive behaviors in a sex-specific manner. We demonstrated that adult male and female offspring of dams maternally exposed from GD7 to PND 14 to an OPFR mixture of TDCPP, TPP, and TCP exhibited altered anxiety and locomotor activity in the OFT, EPM, and LDB compared to sesame seed oil controls. In the OFT, OPFR-exposed females exhibited a decrease in the number of corner entries, and males displayed a reduction in 10-cm center zone time, indicative of anxiolytic and anxiogenic behavior, respectively. OPFR males trended toward an increase in locomotor activity, and females had a decrease on this evaluation. In the EPM, OPFR-exposed males exhibited an anxiolytic-like phenotype compared to male controls. In females, however, we did not detect a difference in treatment groups. Lastly, in the LDB, females regardless of treatment spent increased amounts of time in the transition zone, and both OPFR-exposed males and females had increases in locomotor activity compared to same-sex controls. Collectively, our research suggests a sex-specific effect in anxiety phenotypes that is not due to locomotor activity.
Currently, very little is known about perinatal exposure to OPFRs on adult locomotor activity and anxiety-like behavior. Of the literature that does exist in animal models, results suggest that perinatal exposure to OPFRs alters locomotor and anxiety phenotypes in the adult (Baldwin et al., 2017; Gillera et al., 2019; Oliveri et al., 2015; Patisaul et al., 2013). Yet, this research remains unsettled and includes several species, including fish (Oliveri et al., 2015), rats (Baldwin et al., 2017; Patisaul et al., 2013), and prairie voles (Gillera et al., 2019).
One such study examining the OPFRs TDCPP and TPP in zebrafish, a widely used model to study developmental neurobehavioral toxicology (Bailey et al., 2013; McCollum et al., 2011; Nishimura et al., 2015), revealed alterations in locomotor activity (Oliveri et al., 2015). At 5 hr post fertilization, zebrafish (AB* strain) eggs were exposed to these OPFRs at 0.03 and 0.3 μM doses, and continually dosed every 24 hr until 5 days post fertilization. Exposing zebrafish to 0.3 μM TDCPP induced hyperactivity (increased total distance traveled) in adult (12–14 weeks old) developmentally exposed fish in a 5-min novel tank exploration assay (Oliveri et al., 2015). Although there are species differences, this result would support our findings of a trend in increased male OFT activity. This outcome would not support the decrease in activity seen in our OPFR females in the OFT. However, because Oliveri and colleagues analyzed males and females together, we are unable to make conclusions about sex differences (Oliveri et al., 2015). Moreover, when the authors examined the anxiety parameter in this assay, linear increase in distance from the tank bottom over the 5-min trial, they found that the fish exposed to the 0.3 μM dose of TPP had a smaller rise than in control fish. Typically, when prey fish are placed in a novel tank, fish that appear anxious avoid the more exposed upper regions and spend most of their time at the bottom region of the tank. This suggests an anxiety phenotype in the higher-dosed TPP exposed fish, an effect seen in our male mice developmentally exposed to OPFRs in the OFT. This outcome is not in agreement with our OPFR males exhibiting an anxiolytic phenotype in the EPM. We attribute this disagreement to several factors such as species, type of OPFR used, and apparatus tested. We attribute the novel tank exploration assay more similar to the OFT than the EPM, as bottom dwelling in the tank would most closely match thigmotaxis behavior in an open arena without an ability to escape.
Work by Baldwin and colleagues (Baldwin et al., 2017) is of particular interest as this group investigated developmental exposure (GD 9–18) to FM 550 (0, 300, or 1000 μg/day; oral administration) on adult anxiety-like behavior in male and female Wistar rats. Adult assessment of the EPM (PND 185–192), were similar to our findings such that no effect in anxiety behavior was observed in females at any dose of FM 550 they evaluated. In males, time spent in the open arms and number of open-arm entries were reduced at the 300-μg dose, representative of an anxiogenic effect (Baldwin et al., 2017). This contrasts our own work, as we detected an anxiolytic phenotype in OPFR males. This group did not measure percentage of time, percentage of entries in the open arms, or open-arm end time—measurements in our own study that are thought to be essential assessments of anxiety-like behavior in this task. Baldwin and colleagues also assessed adult (PND 110–120) anxiety behavior in the LDB. Their LDB assessments of anxiety in the study conflict with their EPM assessments in a sex-specific way (Baldwin et al., 2017). In females exposed to the 300-μg FM 550 dose, an increased latency to enter the light zone of the LDB was detected, representative of anxiety. This same FM 550 dose elicited an anxiety response in males in the EPM, but only in females in the LDB. Moreover, no effect in males was observed at any dose on the LDB, and no effects were observed for females in their EPM assessments (Baldwin et al., 2017).
Additional work in the same animal models evaluated one of two doses (100 or 1000 μg; daily, oral administration) of FM 550 from GD 8 through weaning (Patisaul et al., 2013). In adults, anxiety behavior was analyzed via the zero maze (ZM) (PND 90–99) and the EPM (PND 135–143) (Patisaul et al., 2013). In females observed in the ZM, the groups exposed to 100-μg and 1000-μg doses had a reduced number of open-arm entries. In addition, at the 1000-μg dose only, females had a greater latency to enter the open arms than controls. We did not detect an effect in OPFR-exposed females in the similar EPM and observed an anxiolytic effect in these females in our OFT. We attribute this anxiolytic effect in OPFR females to the increase in distance traveled. Given that locomotion and anxiety in the OFT are intrinsically linked, we cannot make determinations of anxiety because we found a significant increase in female locomotion. In males, Patisaul and colleagues observed that exposure to the 100-μg FM 550 dose, males made more ZM open-arm entries than their same-sex counterparts (Patisaul et al., 2013). This finding is similar to our increase in number of open-arm entries observed in OPFR-exposed males in the EPM. Although we did not test this parameter, the authors did detect a trend in ZM reduced latency to enter an open arm in 100-μg exposed males, solidifying our collective finding of anxiolytic OPFR-induced phenotypes in males. The authors also utilized the EPM and observed the opposite effect in anxiety-like behavior in FM 550-exposed females. A dose-dependent decrease and increase were detected in the number of open-arm entries and latency to enter an open arm, respectively. No anxiety effects were observed on the EPM in males. The researchers may have observed differences in these two apparatuses as the ZM may be more sensitive to developmental FM 500 in rats in contrast to the EPM, a finding observed using benzodiazepines (Kulkarni et al., 2008). Alternatively, disparities may have existed due to testing at different ages. From our research we feel that conflicting results in the studies by this group could be attributed to species differences or the usage of FM 550, which does include a FR used in our own research, as well as a mixture of isopropylated triphenylphosphate isomers (ITPs), 2-ethylhexyl-2,3,4, 5-tetrabromobenzoate (TBB), and bis(2-ethylhexyl)- 2,3,4,5-tetrabromophthalate (TBPH) (Patisaul et al., 2013).
We observed locomotor differences in the LDB. Both OPFR-exposed males and females exhibited a significant increase in distance traveled compared to their same-sex oil controls. However, because we were unable to track in the dark portion of the LDB it is not informative for overall locomotor activity. Activity measurements in the LDB would therefore only represent locomotion in the anxiety-promoting light zone. This could be why we observe opposite effects of exposure in females on distance traveled in the OFT and LDB. We observed similar increases in speed in OPFR-exposed mice that are only representative of the light zone. Moreover, the contradictory differences we find in females between LDB speed and OFT distance cannot be directly compared. Recent literature suggests that behavioral analyses of speed and distance can only be made depending on sex (Careau et al., 2012). For example, Careau and colleagues evaluated distance traveled in the OFT and the relationship to speed on a running wheel (Careau et al., 2012). In males, distance was positively correlated with speed, but in females, there was no association between distance and speed (Careau et al., 2012).
Lastly, recent work was conducted in FM 550 developmentally exposed prairie voles (Microtus ochrogaster) (Gillera et al., 2019). Vole dams were exposed to either 500, 1000, or 2000 μg of FM 550 via s.c. injection from GD 0 until birth, and offspring were injected from PND 1 to PND 21. On PND 80, male and female offspring were tested on the OFT (30 min). In regards to locomotor behavior the authors found that females exposed to 500 μg of FM 550 had a reduction in distance traveled compared to their same-sex counterparts (Gillera et al., 2019). Although our OFT time period was only 10 minutes, we also detected a reduction in locomotion in OPFR-exposed females.
Our study has certain limitations. First, our animals were tested in the light phase of their light/dark cycle, a period when animals are typically asleep. During the experimental period, our mouse colony existed in a shared space with other laboratories and we were unable to adjust the light cycle. Thus, we were unable to test in the dark phase, the ideal period to capture behavioral endpoints. In the future, our laboratory plans on using designated animal facility space to utilize a reverse light:dark cycle. Second, many of the behaviors assessed are influenced by the estrous cycle. Due to the nature of our study, all females from a particular dam were tested on the same day, so we were unable to test during a particular cycle stage. Moreover, our results were averaged for each litter, typical methodology for a toxicological study. Lastly, we did not uniformly observe similar anxiety-like behavior across all apparatuses. We attribute these differences to potential order effects of the tests or a possible sensitivity of the EPM to the detection of developmental exposure to our OPFR mixture in comparison to the OFT and LDB.
In conclusion, our study demonstrates sex- and exposure-dependent effects of perinatal OPFR exposure on locomotor and anxiety-like behaviors in male and female adult mice, suggesting developmental neurotoxicity. Future studies should expand on our assessments of anxiety and activity levels in these mice. Collectively, our results support a detrimental role of perinatal OPFR exposure on behavior that is sustained into adulthood. Increased understanding of developmental exposure to OPFRs may bring light to a host of psychiatric and neurodevelopmental disorders that have a sex bias.
Highlights.
Maternal Organophosphate Flame Retardant (OPFR) exposure disrupts adult behavior
Males and females exposed to OPFR are differentially affected on locomotor activity
Males maternally exposed to OPFR exhibit anxiolytic-like behavior
Maternal OPFR exposure produces long-term behavioral changes in the adult offspring
Acknowledgements
The authors wish to thank Katherine Manger for careful editing of the manuscript. This work was supported by the US Department of Agriculture–National Institute of Food and Agriculture (NJ06195) and the National Institutes of Health (R21ES027119 to T.A.R.; P30ES005022 to T.A.R.). S.A. was funded by R25ES020721 and K.W. was funded by T32ES007148.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alaee M, Arias P, Sjödin A, Bergman Å, 2003. An overview of commercially used brominated flame retardants, their applications, their use patterns in different countries/regions and possible modes of release. Environment international 29, 683–689. [DOI] [PubMed] [Google Scholar]
- Archer J, 1973. Tests for emotionality in rats and mice: a review. Animal behaviour 21, 205–235. [DOI] [PubMed] [Google Scholar]
- Bailey J, Oliveri A, Levin ED, 2013. Zebrafish model systems for developmental neurobehavioral toxicology. Birth Defects Research Part C: Embryo Today: Reviews 99, 14–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin KR, Phillips AL, Horman B, Arambula SE, Rebuli ME, Stapleton HM, Patisaul HB, 2017. Sex specific placental accumulation and behavioral effects of developmental Firemaster 550 exposure in Wistar rats. Scientific reports 7, 7118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belcher SM, Cookman CJ, Patisaul HB, Stapleton HM, 2014. In vitro assessment of human nuclear hormone receptor activity and cytotoxicity of the flame retardant mixture FM 550 and its triarylphosphate and brominated components. Toxicology letters 228, 93–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman Å, Heindel JJ, Jobling S, Kidd K, Zoeller TR, Organization WH, 2013. State of the science of endocrine disrupting chemicals 2012: summary for decision-makers. [Google Scholar]
- Bourin M, Hascoët M, 2003. The mouse light/dark box test. European journal of pharmacology 463, 55–65. [DOI] [PubMed] [Google Scholar]
- Butt CM, Hoffman K, Chen A, Lorenzo A, Congleton J, Stapleton HM, 2016. Regional comparison of organophosphate flame retardant (PFR) urinary metabolites and tetrabromobenzoic acid (TBBA) in mother-toddler pairs from California and New Jersey. Environment international 94, 627–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Careau V, Bininda-Emonds OR, Ordonez G, Garland T, 2012. Are voluntary wheel running and open-field behavior correlated in mice? Different answers from comparative and artificial selection approaches. Behavior genetics 42, 830–844. [DOI] [PubMed] [Google Scholar]
- Carignan CC, McClean MD, Cooper EM, Watkins DJ, Fraser AJ, Heiger-Bernays W, Stapleton HM, Webster TF, 2013. Predictors of tris (1, 3-dichloro-2-propyl) phosphate metabolite in the urine of office workers. Environment international 55, 56–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J, 1973. Eta-squared and partial eta-squared in fixed factor ANOVA designs. Educational and psychological measurement 33, 107–112. [Google Scholar]
- Cohen J, 1988. Statistical power analysis for the behavioral sciences. Abingdon. United Kingdom: Routledge. [Google Scholar]
- Cohen J, 2013. Statistical power analysis for the behavioral sciences. Routledge. [Google Scholar]
- Cooper EM, Kroeger G, Davis K, Clark CR, Ferguson PL, Stapleton HM, 2016. Results from screening polyurethane foam based consumer products for flame retardant chemicals: assessing impacts on the change in the furniture flammability standards. Environmental science & technology 50, 10653–10660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daub T, 2004. California-Rogue State or National Leader in Environmental Regulation: An Analysis of California’s Ban of Bromated Flame Retardants. S. Cal. Interdisc. LJ 14, 345. [Google Scholar]
- Dickerson SM, Gore AC, 2007. Estrogenic environmental endocrine-disrupting chemical effects on reproductive neuroendocrine function and dysfunction across the life cycle. Reviews in Endocrine and Metabolic Disorders 8, 143–159. [DOI] [PubMed] [Google Scholar]
- Dodson RE, Perovich LJ, Covaci A, Van den Eede N, Ionas AC, Dirtu AC, Brody JG, Rudel RA, 2012. After the PBDE phase-out: a broad suite of flame retardants in repeat house dust samples from California. Environmental science & technology 46, 13056–13066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giesy JP, Hilscherova K, Jones P, Kannan K, Machala M, 2002. Cell bioassays for detection of aryl hydrocarbon (AhR) and estrogen receptor (ER) mediated activity in environmental samples. Marine Pollution Bulletin 45, 3–16. [DOI] [PubMed] [Google Scholar]
- Gillera SEA, Marinello WP, Horman BM, Phillips AL, Ruis MT, Stapleton HM, Reif DM, Patisaul HB, 2019. Sex-specific effects of perinatal FireMaster® 550 (FM 550) exposure on socioemotional behavior in prairie voles. Neurotoxicology and teratology, 106840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould TD, Dao D, Kovacsics C, 2009. Mood and anxiety related phenotypes in mice. Humana Press. [Google Scholar]
- He C, English K, Baduel C, Thai P, Jagals P, Ware RS, Li Y, Wang X, Sly PD, Mueller JF, 2018. Concentrations of organophosphate flame retardants and plasticizers in urine from young children in Queensland, Australia and associations with environmental and behavioural factors. Environmental research 164, 262–270. [DOI] [PubMed] [Google Scholar]
- Hoffman K, Butt CM, Chen A, Limkakeng AT Jr, Stapleton HM, 2015. High exposure to organophosphate flame retardants in infants: associations with baby products. Environmental science & technology 49, 14554–14559. [DOI] [PubMed] [Google Scholar]
- Kajta M, Wójtowicz AK, 2013. Impact of endocrine-disrupting chemicals on neural development and the onset of neurological disorders. Pharmacological Reports 65, 1632–1639. [DOI] [PubMed] [Google Scholar]
- Kim Y-J, Osako M, Sakai S. i., 2006. Leaching characteristics of polybrominated diphenyl ethers (PBDEs) from flame-retardant plastics. Chemosphere 65, 506–513. [DOI] [PubMed] [Google Scholar]
- Krumm EA, Patel VJ, Tillery TS, Yasrebi A, Shen J, Guo GL, Marco SM, Buckley BT, Roepke TA, 2017. Organophosphate flame-retardants alter adult mouse homeostasis and gene expression in a sex-dependent manner potentially through interactions with ERα. Toxicological Sciences 162, 212–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni SK, Singh K, Bishnoi M, 2008. Comparative behavioural profile of newer antianxiety drugs on different mazes. [PubMed] [Google Scholar]
- Lintelmann J, Katayama A, Kurihara N, Shore L, Wenzel A, 2003. Endocrine disruptors in the environment (IUPAC Technical Report). Pure and Applied Chemistry 75, 631–681. [Google Scholar]
- Liu C, Wang Q, Liang K, Liu J, Zhou B, Zhang X, Liu H, Giesy JP, Yu H, 2013. Effects of tris (1, 3-dichloro-2-propyl) phosphate and triphenyl phosphate on receptor-associated mRNA expression in zebrafish embryos/larvae. Aquatic toxicology 128, 147–157. [DOI] [PubMed] [Google Scholar]
- Liu X, Ji K, Choi K, 2012. Endocrine disruption potentials of organophosphate flame retardants and related mechanisms in H295R and MVLN cell lines and in zebrafish. Aquatic toxicology 114, 173–181. [DOI] [PubMed] [Google Scholar]
- Ma J, Zhu H, Kannan K, 2019. Organophosphorus flame retardants and plasticizers in breast milk from the United States. Environmental science & technology letters 6, 525–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda S, Matsuzawa D, Ishii D, Tomizawa H, Sutoh C, Nakazawa K, Amano K, Sajiki J, Shimizu E, 2012. Effects of perinatal exposure to low dose of bisphenol A on anxiety like behavior and dopamine metabolites in brain. Progress in Neuro-Psychopharmacology and Biological Psychiatry 39, 273–279. [DOI] [PubMed] [Google Scholar]
- McCollum CW, Ducharme NA, Bondesson M, Gustafsson JA, 2011. Developmental toxicity screening in zebrafish. Birth Defects Research Part C: Embryo Today: Reviews 93, 67–114. [DOI] [PubMed] [Google Scholar]
- Nishimura Y, Murakami S, Ashikawa Y, Sasagawa S, Umemoto N, Shimada Y, Tanaka T, 2015. Zebrafish as a systems toxicology model for developmental neurotoxicity testing. Congenital anomalies 55, 1–16. [DOI] [PubMed] [Google Scholar]
- Oliveri A, Bailey J, Levin ED, 2015. Developmental exposure to organophosphate flame retardants causes behavioral effects in larval and adult zebrafish. Neurotoxicology and teratology 52, 220–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patisaul HB, Bateman HL, 2008. Neonatal exposure to endocrine active compounds or an ERβ agonist increases adult anxiety and aggression in gonadally intact male rats. Hormones and behavior 53, 580–588. [DOI] [PubMed] [Google Scholar]
- Patisaul HB, Roberts SC, Mabrey N, McCaffrey KA, Gear RB, Braun J, Belcher SM, Stapleton HM, 2013. Accumulation and endocrine disrupting effects of the flame retardant mixture Firemaster® 550 in rats: an exploratory assessment. Journal of biochemical and molecular toxicology 27, 124–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera F, Nolte ELR, Wang Y, Margolis AE, Calafat AM, Wang S, Garcia W, Hoepner LA, Peterson BS, Rauh V, 2016. Bisphenol A exposure and symptoms of anxiety and depression among inner city children at 10–12 years of age. Environmental research 151, 195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera F, Vishnevetsky J, Herbstman JB, Calafat AM, Xiong W, Rauh V, Wang S, 2012. Prenatal bisphenol a exposure and child behavior in an inner-city cohort. Environmental health perspectives 120, 1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips AL, Chen A, Rock KD, Horman B, Patisaul HB, Stapleton HM, 2016. Editor’s highlight: transplacental and lactational transfer of Firemaster® 550 components in dosed Wistar rats. Toxicological Sciences 153, 246–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roen EL, Wang Y, Calafat AM, Wang S, Margolis A, Herbstman J, Hoepner LA, Rauh V, Perera FP, 2015. Bisphenol A exposure and behavioral problems among inner city children at 7–9 years of age. Environmental research 142, 739–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers JA, Metz L, Yong VW, 2013. endocrine disrupting chemicals and immune responses: a focus on bisphenol-A and its potential mechanisms. Molecular immunology 53, 421–430. [DOI] [PubMed] [Google Scholar]
- Ryan BC, Vandenbergh JG, 2006. Developmental exposure to environmental estrogens alters anxiety and spatial memory in female mice. Hormones and behavior 50, 85–93. [DOI] [PubMed] [Google Scholar]
- Sjödin A, Patterson DG, Bergman Å, 2001. Brominated flame retardants in serum from US blood donors. Environmental science & technology 35, 3830–3833. [DOI] [PubMed] [Google Scholar]
- Stapleton HM, Kelly SM, Allen JG, McClean MD, Webster TF, 2008. Measurement of polybrominated diphenyl ethers on hand wipes: estimating exposure from hand-to-mouth contact. Environmental science & technology 42, 3329–3334. [DOI] [PubMed] [Google Scholar]
- Stapleton HM, Klosterhaus S, Eagle S, Fuh J, Meeker JD, Blum A, Webster TF, 2009. Detection of organophosphate flame retardants in furniture foam and US house dust. Environmental science & technology 43, 7490–7495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton HM, Klosterhaus S, Keller A, Ferguson PL, van Bergen S, Cooper E, Webster TF, Blum A, 2011. Identification of flame retardants in polyurethane foam collected from baby products. Environmental science & technology 45, 5323–5331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton HM, Misenheimer J, Hoffman K, Webster TF, 2014. Flame retardant associations between children’s handwipes and house dust. Chemosphere 116, 54–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan GM, Feinn R, 2012. Using effect size—or why the P value is not enough. Journal of graduate medical education 4, 279–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian YH, Baek JH, Lee SY, Jang CG, 2010. Prenatal and postnatal exposure to bisphenol a induces anxiolytic behaviors and cognitive deficits in mice. Synapse 64, 432–439. [DOI] [PubMed] [Google Scholar]
- Walf AA, Frye CA, 2007. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nature protocols 2, 322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong KH, Durrani TS, 2017. Exposures to endocrine disrupting chemicals in consumer products—a guide for pediatricians. Current problems in pediatric and adolescent health care 47, 107–118. [DOI] [PubMed] [Google Scholar]
- Xu X, Hong X, Xie L, Li T, Yang Y, Zhang Q, Zhang G, Liu X, 2012. Gestational and lactational exposure to bisphenol-A affects anxiety-and depression-like behaviors in mice. Hormones and behavior 62, 480–490. [DOI] [PubMed] [Google Scholar]
- Xue J, Zartarian V, Moya J, Freeman N, Beamer P, Black K, Tulve N, Shalat S, 2007. A meta-analysis of children’s hand-to-mouth frequency data for estimating nondietary ingestion exposure. Risk Analysis: An International Journal 27, 411–420. [DOI] [PubMed] [Google Scholar]
- Zoeller RT, Brown TR, Doan LL, Gore AC, Skakkebaek N, Soto A, Woodruff T, Vom Saal F, 2012. Endocrine-disrupting chemicals and public health protection: a statement of principles from The Endocrine Society. Endocrinology 153, 4097–4110. [DOI] [PMC free article] [PubMed] [Google Scholar]