FIG. 9.
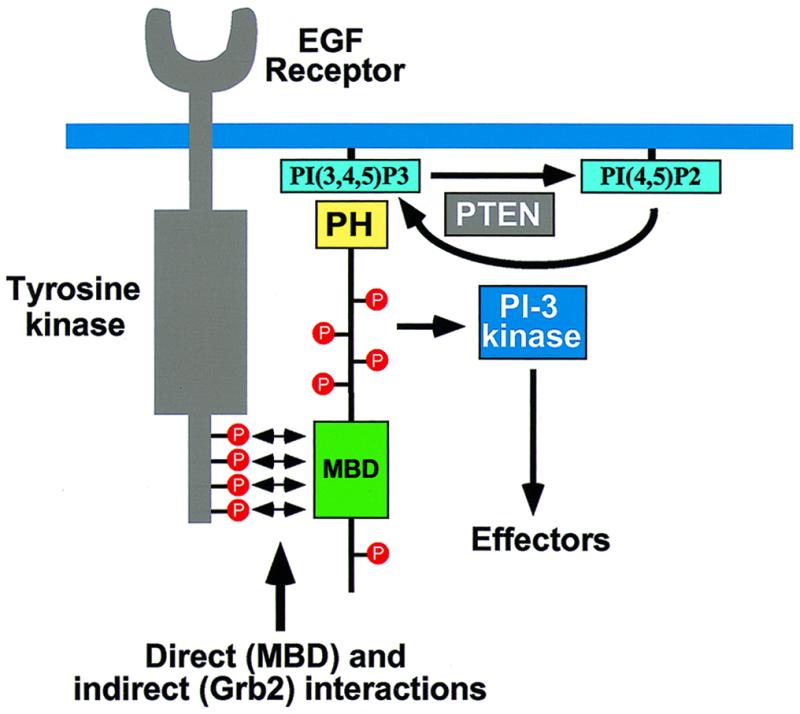
Model outlining the role of Gab1 in receptor tyrosine kinase signaling. Gab1 is recruited to the plasma membrane in response to growth factor stimulation through the concerted action of binding of its MBD to the EGFR directly and through Grb2 and binding of its PH domain to the products of PI-3 kinase. Tyrosine phosphorylation of Gab1 by EGFR leads to recruitment and activation of signaling molecules, including PI-3 kinase. Activation of PI-3 kinase in turn leads to the generation of phosphoinositides that enhance downstream signaling as well as initiate a positive feedback by recruiting more Gab1 molecules to the EGFR. Conversion of PtdIns(3,4,5)P3 to PtdIns(4,5)P2 by PTEN allows for dissociation of Gab1 from the plasma membrane and termination of signaling through Gab1.
