Figure 1.
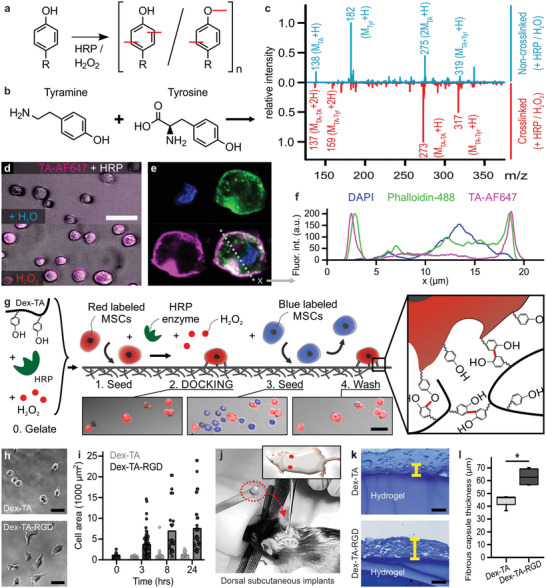
Discrete inducible tethering of cells and non‐cell‐adhesive materials via enzyme‐mediated oxidative crosslinking. a) Phenolic moieties can be enzymatically coupled and oligomerized using horseradish peroxidase (HRP) and hydrogen peroxide (H2O2) via the formation of C—C and C—O bonds. b,c) ESI‐MS confirmed the enzymatic crosslinking (i.e., red peaks) of tyramine to tyramine ([MTA–TA + H]+: 273, [MTA–TA + 2H]2+: 137) and tyramine to tyrosine ([MTA–Tyr + H]+: 317, [MTA–Tyr + 2H]2+: 159). The blue plot indicates the control experiment (i.e., with dH2O instead of H2O2). d) Enzyme‐mediated crosslinking could also be leveraged to couple fluorescently labeled tyramine (TA‐AF647, magenta) directly onto cells, e,f) thereby predominantly staining pericellularly as shown using confocal microscopy. Cells were stained with phalloidin (green) and DAPI (blue). g) Endowing dextran with tyramine moieties enabled the formation of Dex‐TA hydrogel substrates (i.e., “0. Gelate”), onto which (red labeled) cells could be seeded (i.e., “1. Seed”) and tethered using an enzymatic post‐cure (i.e., “2. DOCKING”). In contrast, (blue labeled) cells that were seeded (i.e., “3. Seed”) but not tethered to the same Dex‐TA substrate were easily washed away (i.e., 4. Wash'). h,i) MSCs adhered and spread on Dex‐TA‐RGD, but not on Dex‐TA substrates. j–l) Dorsal subcutaneous implantation of Dex‐TA hydrogel disks in C57BL/6 mice revealed significantly less fibrotic capsule formation as compared to disks made of Dex‐TA‐RGD. The yellow lines indicate representative fibrotic capsule thickness measurements of Toluidine Blue stained sections. Boxes indicate 25–75 percentiles, lines indicate medians, whiskers indicate min–max, n = 4, significance is indicated (* p < 0.05; Mann–Whitney). The scale bars indicate 50 µm.
