Figure 4.
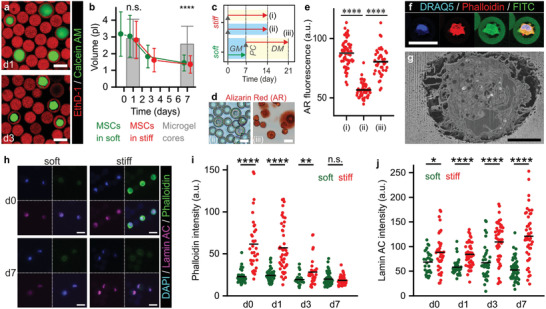
Mechanotransduction in 3D‐tethered MSCs is not dependent on cell volume changes and spreading. a) Confocal microscopic analysis of live/dead stained microencapsulated MSCs showed shrinkage of cells during culture in microgels. b) Time‐lapse quantification of cell volumes revealed that MSCs in both soft and stiff microgels significantly reduced volumes during in vitro culture as compared to the microgel core volume. c) Experimental plan to assess the effect of MSC shrinkage on their osteogenic differentiation potential. d,e) Assessing calcified extracellular matrix using Alizarin Red staining revealed that MSCs cultured for 1 week in soft microgels and GM followed by a stiffening enzymatic post‐cure and 2 weeks culture in osteogenic DM (iii) remained their osteogenic potential, when compared to 2 weeks culture in stiff microgels and osteogenic DM (i), or GM (ii). f) Confocal microscopy and g) FIB/SEM revealed that shrunk cells are still attached to the Dex‐TA microgel interior. Phalloidin staining intensity was artificially boosted. h–j) Time‐lapse confocal imaging of F‐actin (i.e., phalloidin staining) and lamin A/C expression in MSCs in soft and stiff microgels during 1 week of culture in growth medium (GM). The lines in dot plots indicate means, the error bars indicate ± standard deviation, n ≥ 29 (b), n ≥ 30 (c), n ≥ 120 (e), and n ≥ 40 (h); significance is indicated (**** p < 0.0001, ** p < 0.01, * p < 0.05, “n.s.” p > 0.05, Mann–Whitney). The white scale bars indicate 25 µm and the black scale bar indicates 5 µm.
