Fig. 2 |. Axon growth and innervation of peripheral targets.
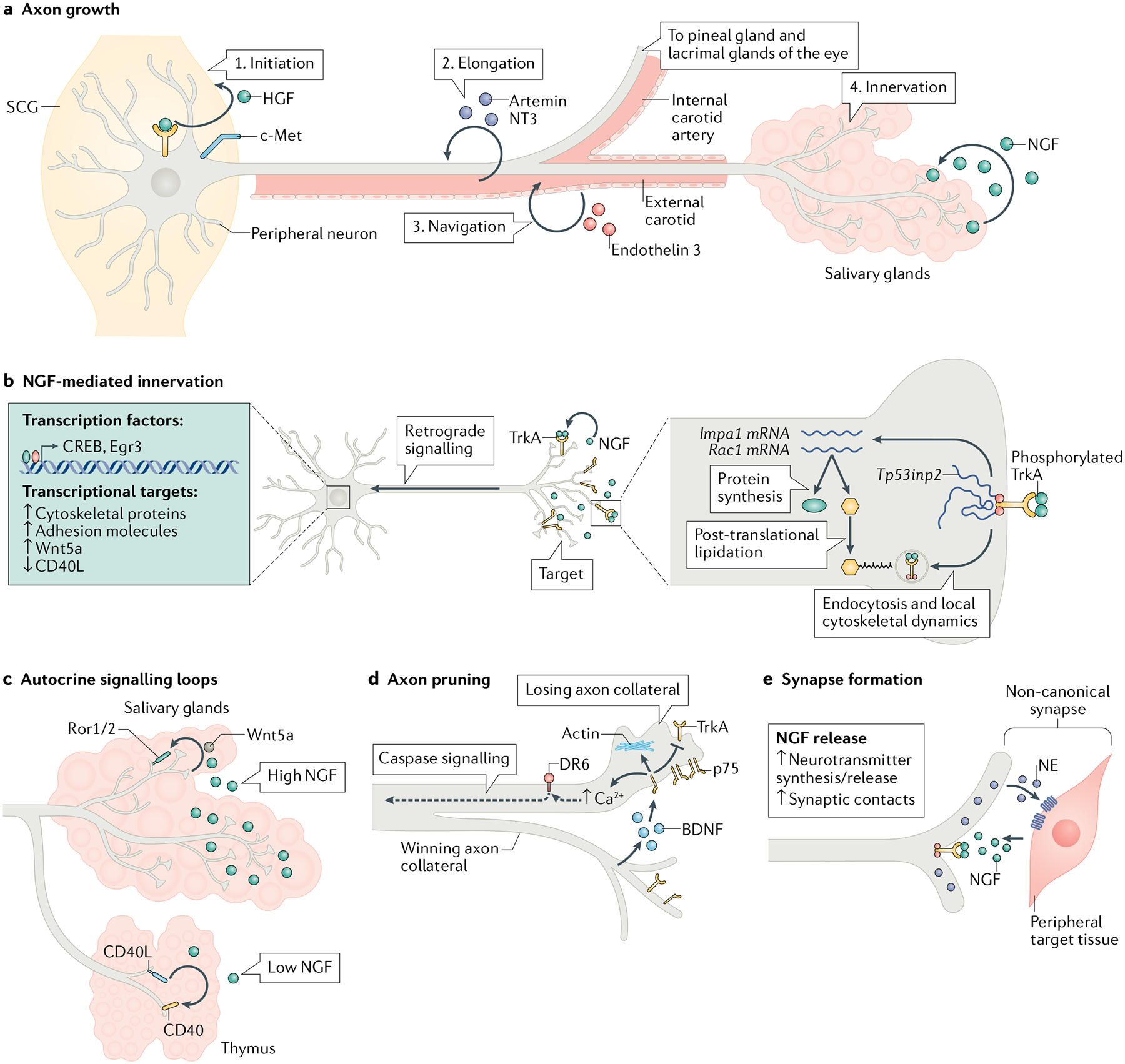
a | Target innervation is a carefully orchestrated process that encompasses axon specification, elongation, pathfinding, and branching in end-organs and involves the combined effects of autocrine and paracrine secreted factors. Autocrine hepatocyte growth factor (HGF) signalling initiates axon extension (1) and axons extend along blood vessels in response to vascular-derived guidance signals, artemin, neurotrophin 3 (NT3) and endothelin 3 (2, 3). Axons undergo terminal branching and growth to fully innervate NGF-expressing final targets (4). b | Target-derived nerve growth factor (NGF) promotes target innervation by activating local cytoskeletal and endocytic pathways in axons and retrogradely regulating transcriptional events in cell bodies and/or nuclei. NGF-mediated axon growth also relies on local protein synthesis of effectors, including Impa1 and Rac1, and also post-translational lipidation of newly synthesized proteins in distal axons. Tp53inp2 is an axonally abundant mRNA that mediates NGF-dependent growth in a non-coding manner. Retrograde NGF signalling via axonal transport of TrkA-signalling endosomes activates transcription factors and the expression of downstream target genes essential for long-term axon growth and target innervation. c | NGF signalling regulates autocrine signalling loops to promote innervation of peripheral organs. NGF induces Wnt5a synthesis in sympathetic neurons and autocrine Wnt signalling through Ror1/2 receptors promotes axon branching. In tissues with low NGF expression, such as the thymus, autocrine CD40L signalling via the CD40 receptor promotes axon branching and innervation. This pathway is downregulated when axons encounter high NGF levels in tissues such as the salivary glands. d | Axon pruning occurs by competitive collateral elimination mediated by the actions of the p75 and DR6 receptors. Activity-dependent brain-derived neurotrophic factor (BDNF) release by ‘winning’ axons activates p75 to antagonize TrkA trophic signalling in ‘losing’ axons. p75 also promotes actin remodelling and intracellular Ca2+ increase to induce degenerative signalling. The actions of DR6 and axonal caspase signalling are also required for axon loss. e | Postganglionic sympathetic neurons form non-canonical synapses with peripheral tissues. Sympathetic synapses resemble bouton like-structures loaded with norepinephrine (NE). The formation of synaptic contacts between sympathetic neurons and peripheral tissue as well as neurotransmitter synthesis and release are regulated by NGF signalling. Egr3, early growth response 3; SCG, superior cervical ganglia.
