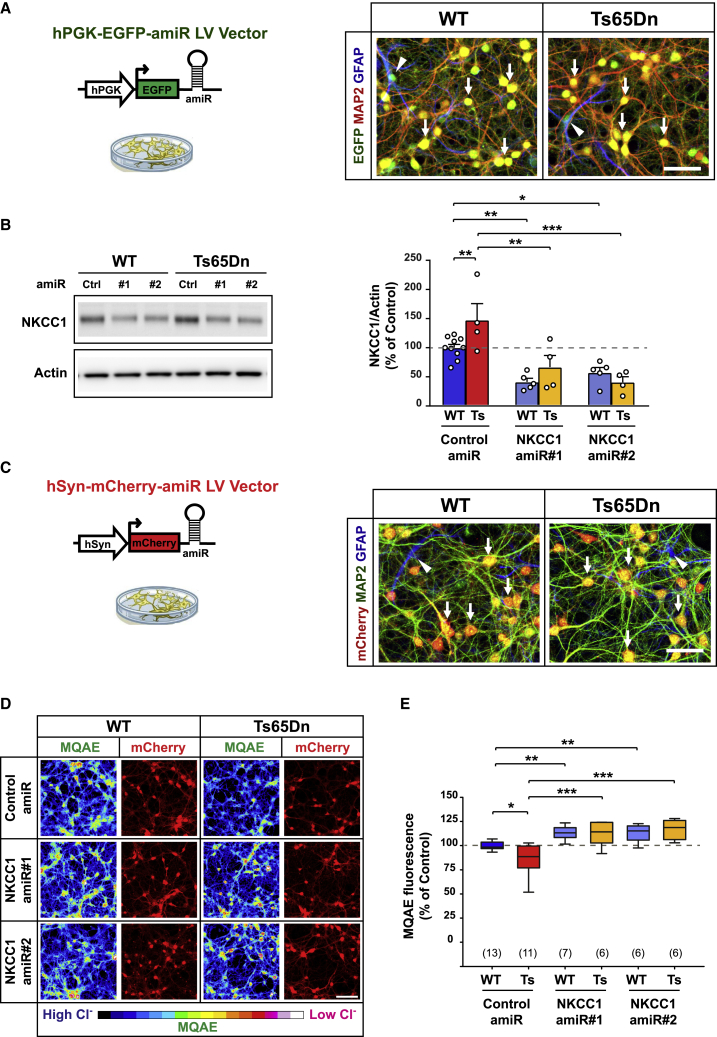
Figure 1.
Anti-NKCC1 amiR treatment rescues intracellular chloride concentration in Ts65Dn neurons in cultures
(A) Left: schematic representation of the lentiviral vector (LV) expressing either control or NKCC1 amiRs from the ubiquitous hPGK1 promoter. Right: representative immunofluorescent images of LV-transduced WT and Ts65Dn hippocampal cultures at 15 DIVs expressing EGFP (green) and immunostained for the neuronal marker MAP2 (red) and the astrocytic marker GFAP (blue). Arrows indicate EGFP and MAP2 double-positive neurons, whereas arrowheads indicate EGFP and GFAP double-positive astrocytes. Scale bar: 50 μm. (B) Left: representative immunoblot for NKCC1 in protein extracts from LV-transduced WT and Ts65Dn neurons at 15 DIVs. Right: quantification of NKCC1 protein (expressed as the percentage of WT neurons transduced with control amiR) showed increased NKCC1 expression in Ts65Dn compared with that of WT neurons. Expression of amiRs 1 and 2 induced significant NKCC1 knockdown in both WT and Ts65Dn neurons. Actin was used as an internal standard. (C) Left: schematic representation of the LVs expressing either control or NKCC1 amiRs from the neuron-specific hSyn promoter. Right: representative immunofluorescent images of LV-transduced WT and Ts65Dn hippocampal cultures at 15 DIVs expressing mCherry (red), and immunostained for the neuronal marker MAP2 (green) and the astrocytic marker GFAP (blue). Arrows indicate mCherry and MAP2 double-positive neurons, whereas arrowheads indicate GFAP+/mCherry-negative astrocytes. Scale bar: 50 μm. (D) Representative images of LV-transduced WT and Ts65Dn hippocampal neurons at 15 DIV (expressing mCherry; red) during imaging experiments with the chloride-sensitive dye MQAE (pseudo-colors). Fluorescent intensity of the dye (color-coded on the bottom) is inversely proportional to [Cl−]i. Scale bar: 100 μm. (E) Quantification of [Cl−]i with MQAE (expressed as the percentage of WT neurons transduced with control amiR) showed lower fluorescent intensity (i.e., greater [Cl−]i) in Ts65Dn compared with that of WT neurons. NKCC1 knockdown by amiRs 1 or 2 significantly increased MQAE fluorescent intensity (i.e., lower [Cl−]i) in both WT and Ts65Dn neurons. Data in (B) are means ± SEM; dots indicate values of individual samples (obtained from five independent neuronal cultures). Boxplots in (E) indicate median and 25th–75th percentiles, whiskers represent the 5th–95th percentiles, and numbers in parentheses indicate the number of analyzed samples (obtained from six independent neuronal cultures). ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, Tukey post hoc test after two-way ANOVA.