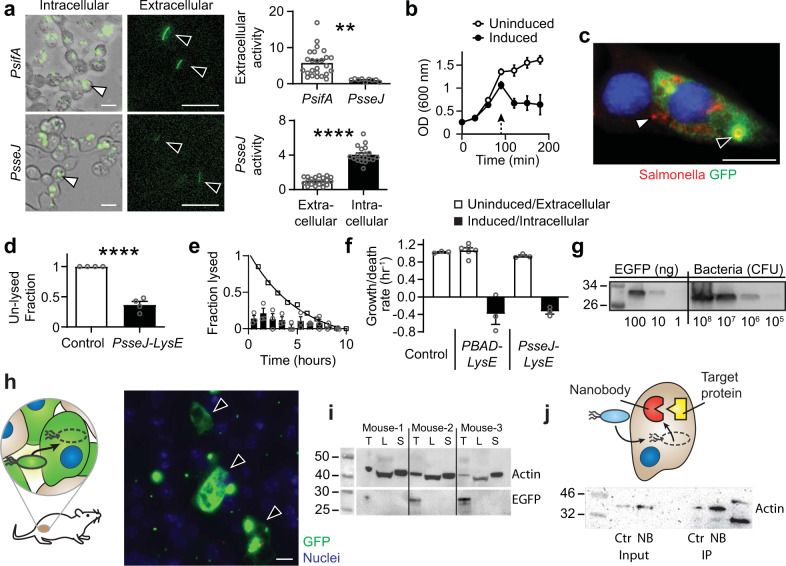
Fig. 2. Design of ID Salmonella to release protein into cells.
a Salmonella with the PsifA-GFP or PsseJ-GFP reporter constructs both expressed GFP after invasion (white arrows). Extracellular expression (black arrows) from PsseJ-GFP was less than PsifA-GFP (P = 0.005; n = 27 for PsifA and n = 9 for PsseJ). The intracellular activity of the PsseJ promoter was four times greater than extracellular activity (P < 0.0001; n = 20). b Induction of PBAD-LysE at 96 h (arrow) induced bacteria lysis (n = 3). c When administered to MCF7 cancer cells, Salmonella (red, white arrow) with PsseJ-LysE and Plac-GFP delivered GFP (green, black arrow) into the cellular cytoplasm. Only released, and not intrabacterial GFP was stained. d Most intracellular Salmonella with PsseJ-LysE lysed, which was significantly greater than PsseJ-GFP controls (P < 0.0001; n = 4). e Lysis of intracellular PsseJ-LysE Salmonella occurred for 10 h after invasion (n = 3). The fraction of MCF7 cancer cells in which Salmonella lysed (disappeared) per hour (bars) was relatively constant. The cumulative fraction of cells with intact bacteria (open circles) decreased exponentially. f In liquid culture, PBAD-lysE and PsseJ-LysE Salmonella grew at similar rates as nontransformed controls (white bars). When intracellular, PsseJ-LysE Salmonella, lysed at a similar rate as induced PBAD-lysE Salmonella in culture (black bars; n = 3 for all conditions except n = 6 for uninduced PBAD-lysE). g The EGFP content of PsseJ-LysE Salmonella was determined by immunoblot. Lysed bacteria at 106, 107, 108, and 109 colony forming units (CFU) per well (four right lanes) were compared to EGFP standards at three concentrations: 1, 10, and 100 ng/well (three left lanes). h Ninety-six hours after intratumoral injection of 2 × 106 bacteria/mouse to BALB/c mice with subcutaneous 4T1 tumors (n = 3), ID Salmonella (which utilizes PsseJ-LysE) delivered GFP into cancer cells (arrows). i In the same mice, delivered GFP was present in extracts from tumors (T), but not livers (L) or spleens (S). Actin was used as a loading control. j Anti-actin nanobody (NB) and GFP (Ctr) was delivered into 4T1 cancer cells with ID Salmonella. Beta-actin was immuno-precipitated with delivered nanobody and was enriched 2.5 times compared to controls. Data are shown as means ± SEM. Statistical comparisons in a and d are two-tailed, unpaired Student’s t-tests with asterisks indicating significance (**P < 0.01; ****P < 0.0001). Images in a, left and c are representative of 20 and 10 independent biological samples. The immunoblot in j is from a single experiment and the immunoblots in g and i are each representative of two independent experiments with similar results. Scale bars in a, c, and h are 10 µm.