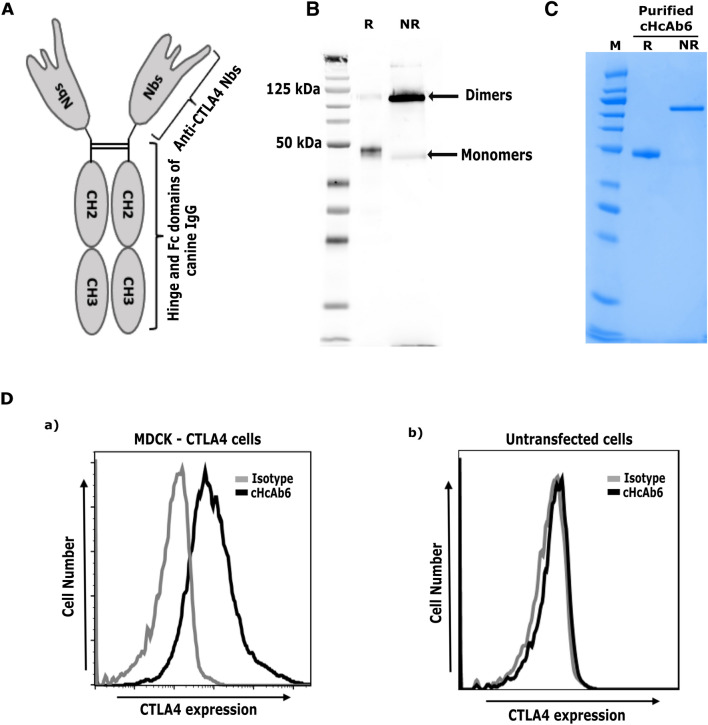
Figure 3.
Development of Nb-based anti-CTLA4 heavy chain only antibody (HcAb). (A) Predicted structure of cHcAb6. The DNA sequence coding for the cNb6 was genetically fused to the hinge and Fc domains of canine IgG (subclass B). (B) The cHcAb6 form dimers as demonstrated by western blotting. The cHcAb6 was expected to form dimers via hinge and Fc domains of canine IgG. The cHcAb6 protein, expressed in ExpiCHO-S cells, was resolved under reducing and non-reducing conditions and detected by anti-IgG Fc antibody. The cHcAb6, as expected, forms dimers of ~ 83 kDa under non-reducing conditions. (R-reducing condition, NR-non reducing). (C) Purity of cHcAb6 assessed by SDS-PAGE. The cHcAb6 was expressed and purified from the ExpiCHO-S cells by affinity (Protein A) and size-exclusion chromatography. The purified cHcAb6 was resolved under reducing (R) and non-reducing (NR) condition and stained with GelCode Blue Stain. (D) Binding of cHcAb6 to cells expressing canine CTLA4 demonstrated by flow cytometry. MDCK cells transiently expressing CTLA4 were stained with cHcAb6, washed, and bound cHcAb6 was detected using anti-Fc-750 antibody by flow cytometry. cHcAb6 does not bind to untransfected cells.