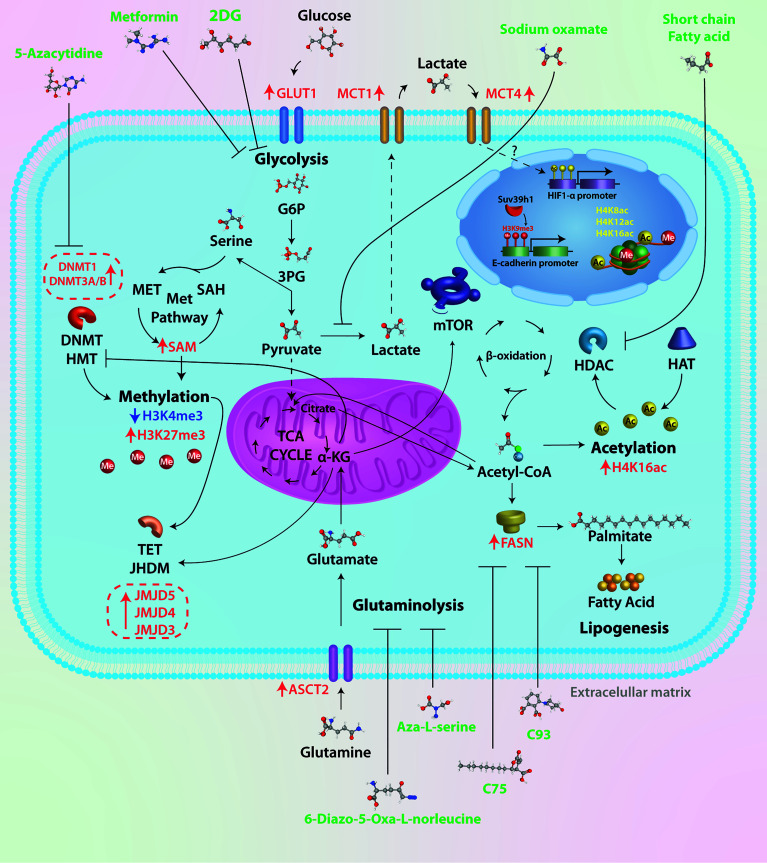
Figure 1.
Therapeutic targets of tumor metabolism. Tumor cells have a high glycolytic rate and overexpress key enzymes of glucose metabolism. Several drugs have been used in preclinical trials to test their effectiveness, such as 2DG, a glucose analog whose metabolic product (2-DG-P) is unable to be metabolized, therefore inhibiting glycolysis. Oxamic acid is a competitive inhibitor of LDHA, an enzyme overexpressed in breast cancer cells; the result of this inhibition is lower levels of lactate, a critical oncometabolite for cell migration and tumor progression. In tumor cells, approximately 90% of glucose is metabolized to lactate; to replenish the deficit of carbon molecules, there is an increase in glutamine metabolism. The ASCT2 transporter is overexpressed in breast cancer; glutamine is metabolized in the cytosol to glutamate and subsequently transported to the mitochondrial matrix and incorporated into TCA in the form of α-KG. The antitumor effect of two glutamine analogs (6-diazo-5-oxa-L-norleucine and Aza-L-serine) has been shown. Acetyl-CoA is a central molecule in metabolism as it participates in catabolic (glycolysis and beta-oxidation) and anabolic reactions (lipogenesis, steroid synthesis, acetylcholine synthesis, etc.). In addition to this crucial role in cellular metabolism, Acetyl-CoA is the sole donor of acetyl groups for the acetylation of proteins and particularly histones. Histone acetylation is catalyzed by histone acetyltransferases (HATs), whereas removal of the acetyl group is mediated by histone deacetylases (HDACs). It has been shown that short-chain fatty acids such as valproic acid and butyrate, among others, can inhibit HDACs. Increased levels of acetyl-CoA promote fatty acid synthesis associated with FASN overexpression. Different inhibitors of the key enzyme in fatty acid synthesis have been used (C75, C93, and orlistat, among others), which are inhibitors of the thioesterase domain of fatty acid synthase (FASN). The epigenetic mechanism classically described is DNA methylation. The pathway that supplies methyl groups for both DNA methylation and histone and protein methylation is the one-carbon (1C) pathway metabolism, in which two distinct pathways, the folate and methionine cycle, converge, resulting in the product S-adenosylmethionine. The epigenetic mechanism classically described is DNA methylation. While no inhibitors of these metabolic pathways have been identified, several molecules have been used to inhibit the activity of enzymes involved in DNA methylation (DNMT1, DNMT3/B).