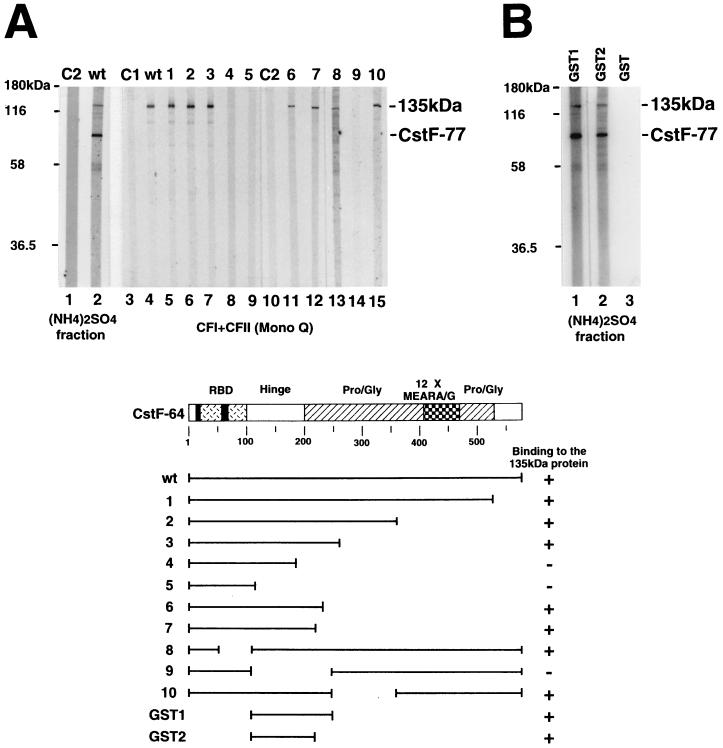
FIG. 3.
The hinge domain of CstF-64 is necessary and sufficient for its binding to a 135-kDa protein. (A) The hinge domain of CstF-64 is necessary for binding to a 135-kDa nuclear protein. The (NH4)2SO4 fraction (20 to 40% saturation) derived from HeLa cell nuclear extract (lanes 1 and 2) and a CFI- and CFII-containing fraction obtained by Mono Q chromatography (lanes 3 to 15) were resolved by SDS-PAGE, transferred to a nitrocellulose membrane, and probed with 35S-labeled wild-type (wt, lanes 2 and 4) or mutant (deletions 1 to 10, lanes 5 to 9 and 11 to 15) CstF-64. 35S-labeled brome mosaic virus proteins (C1, lane 3) and luciferase (C2, lanes 1 and 10) were used as negative controls. Diagrams of wild-type and mutant CstF-64 proteins are shown at the bottom. (B) The hinge domain of CstF-64 is sufficient for binding the 135-kDa protein. Proteins in the (NH4)2SO4 fraction used in panel A were transferred onto a nitrocellulose membrane and probed with 32P-labeled GST (lane 3) or GST fusion (lanes 1 and 2) proteins.