Figure 5.
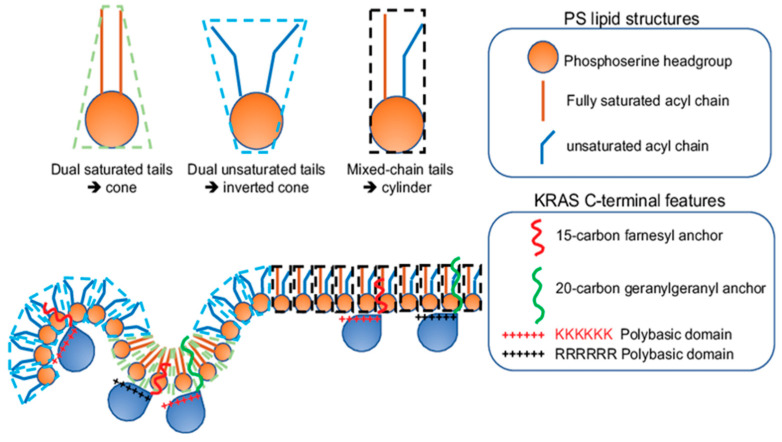
KRAS polybasic domain mutants with equivalent charges possess distinct preferences for membrane curvature. KRASG12V with the original farnesylated hexa-lysine or the geranylgeranylated hexa-arginine prefers to form nanoclusters on flatter membranes with low curvature. On the other hand, KRASG12V with the farnesylated hexa-arginine or the geranylgeranylated hexa-lysine polybasic domain favors to interact with more curved membranes. The ability of these KRAS polybasic domain mutants to selectively sort distinct PS species with different packing geometries contributes to the distinct membrane curvature sensing capabilities of KRAS.