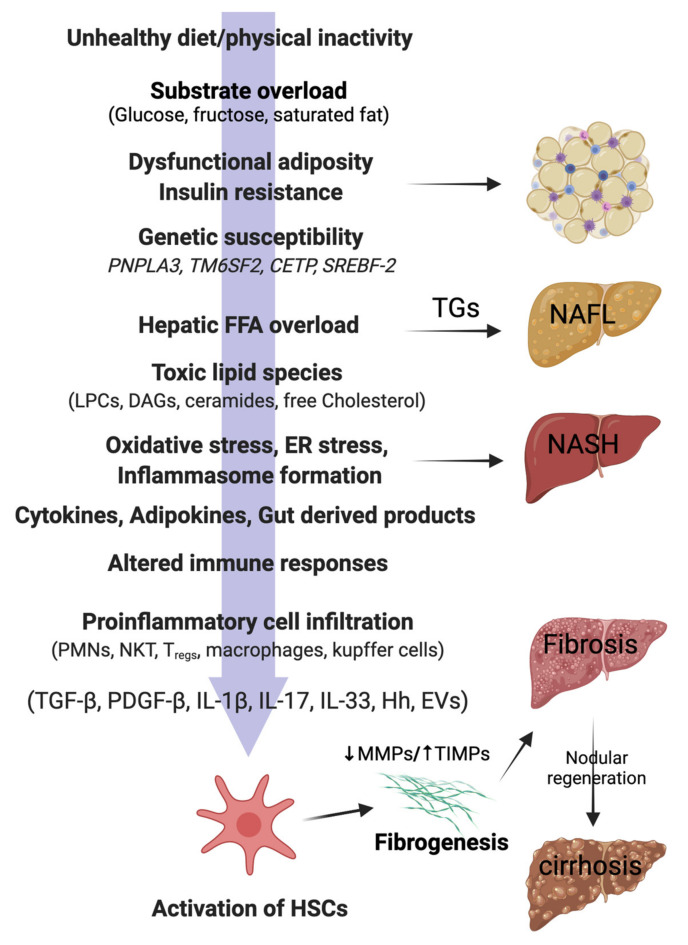
Figure 1.
Pathophysiology of NAFLD in lean individuals. In genetically predisposed (PNPLA3, TM6SF2) lean or non-obese individuals, free fatty acids (FFAs), mainly derived from the visceral adipose tissue, are taken up by the liver. Hepatic FFA accumulation can be influenced by the presence of insulin resistance, dysfunctional adiposity, and/or and lifestyle habits (unhealthy diets, physical inactivity). Excess of dietary sugars (glucose, fructose) are converted into FFAs by de novo lipogenesis (DNL). FFAs in the liver mitochondria undergo β-oxidation or are converted back into triglycerides (TGs) for export to the circulation via VLDL. Overwhelming of FFA disposal mechanisms leads to accumulation of TGs as lipid droplets in the hepatocytes (non-alcoholic fatty liver; NAFL). When the FFA pool expands further, cytotoxic lipid species (e.g., LPCs, DAGs, and ceramides) are produced, which ultimately mediate oxidative stress, endoplasmic reticulum (ER) stress, and inflammasome activation. These pathological processes lead to hepatocellular injury, inflammatory cell recruitment, and apoptosis/necroptosis to produce the histological phenotype of non-alcoholic steatohepatitis (NASH). Major modulators of the hepatocellular response to lipotoxic stress may include the gut microbiota products; a variety of cytokines, chemokines, and adipokines; free cholesterol; uric acid; and possibly periodic hypoxia caused by obstructive sleep apnea. Overinduction of inflammatory processes then stimulates hepatic stellate cells (HSC) and activates fibrogenesis. HSC activation is the final common pathway for a diverse group of signals, such as transforming growth factor-beta (TGF-β), platelet-derived growth factor-beta (PDGF-β), interleukins (ILs), hedgehog ligands (Hhs), and extracellular vesicles (EVs). Increased tissue inhibitors of matrix metalloproteinases (TIMPs) cause inhibition of matrix metalloproteinases (MMPs), thereby leading to net gain of fibrosis tissue by the liver. Excessive and disorganized fibrous tissue causes disruption of hepatocellular architecture and nodule formation, leading to cirrhosis of the liver. PNPLA3, patatin-like phospholipase domain containing 3; TM6SF2, Transmembrane 6 superfamily 2; CETP, Cholesteryl ester transfer protein; SREBF-2, sterol regulatory element binding transcription factor 2; LPCs, lysophosphatidylcholines; DAGs, diacyl glycerols; PMNs, polymorphonuclear leucocytes; NKTs, natural killer T cells; Tregs, regulatory T cells.