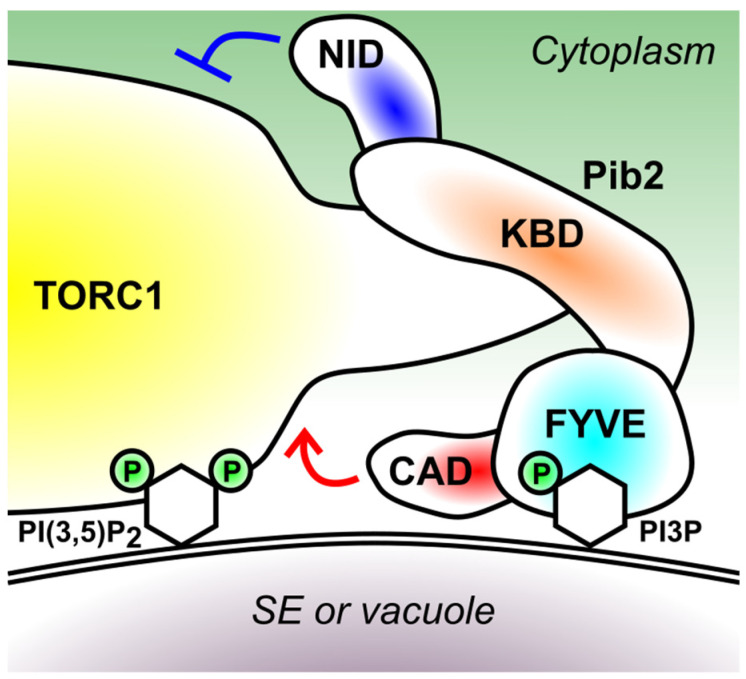
Figure 3.
The domain structure of Pib2 and a model of its membrane recruitment. Pib2 is recruited to the surface of signaling endosomes (SE) and vacuoles via the interaction between its FYVE domain and the membrane lipid PI3P. The KBD domain mediates the interaction with the Kog1 subunit of TORC1, which in turn interacts with the membrane lipid PI(3,5)P2. ‘P’ denotes phosphorylation on the inositol rings (depicted as hexagons) of PI3P and PI(3,5)P2. The CAD and NID domain of Pib2 activates and inhibits TORC1, respectively.