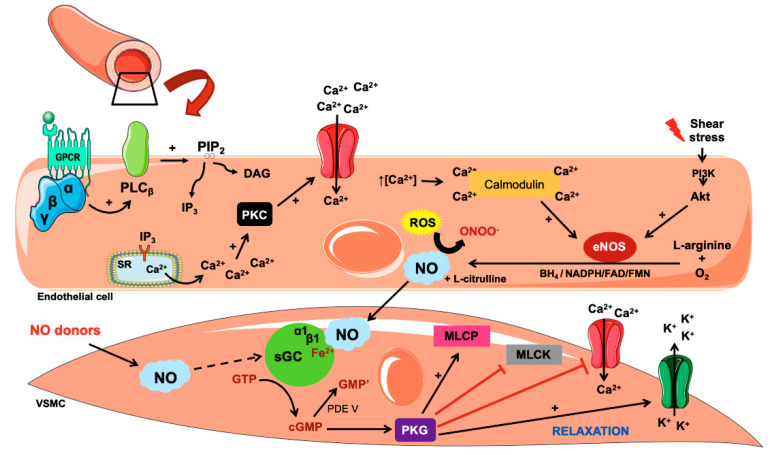
Figure 1.
Nitric oxide (NO) induces relaxation in vascular smooth muscle cells (VSMC). The activation of G-protein coupled receptor (GPCR) stimulates phospholipase C (PLC), which is responsible for cleavage of membrane phospholipids to diacylglycerol (DAG) and inositol 1,4,5-trisphosphate (IP3). The latter binds to the IP3 receptor in sarcoplasmic reticulum (SR) to promote Ca2+ extrusion, which, together with DAG, evokes Ca2+ influx through voltage-operated Ca2+ channels (Cav) at the cellular membrane. The linkage of Ca2+ to calmodulin promotes endothelial nitric oxide synthase (eNOS) activation, which, in turn, triggers the formation of NO and citrulline from arginine and O2. This enzyme requires cofactors such as nicotinamide adenine dinucleotide phosphate (NADPH), flavin adenine dinucleotide (FAD), flavin mononucleotide (FMN), and tetrahydrobiopterin (BH4). In case of an increase in reactive oxygen species (ROS), they react with NO and induce peroxynitrite (ONOO−) production. NO spreads to VSMC where it binds to soluble guanylyl cyclase (sGC) and causes the formation of 3,5-cyclic guanosine monophosphate (cGMP), which stimulates the cGMP-protein kinase G (PKG). This kinase negatively regulates the Cav and myosin light chain kinase (MLCK) and activates potassium channels and the myosin light chain phosphatase (MLCP). Altogether, these effects promote the relaxation of VSMC. In addition, NO can be produced from a GPCR-independent mechanism. The shear stress promotes activation of the PI3K/AKT pathway, which stimulates eNOS activation and subsequent NO production.