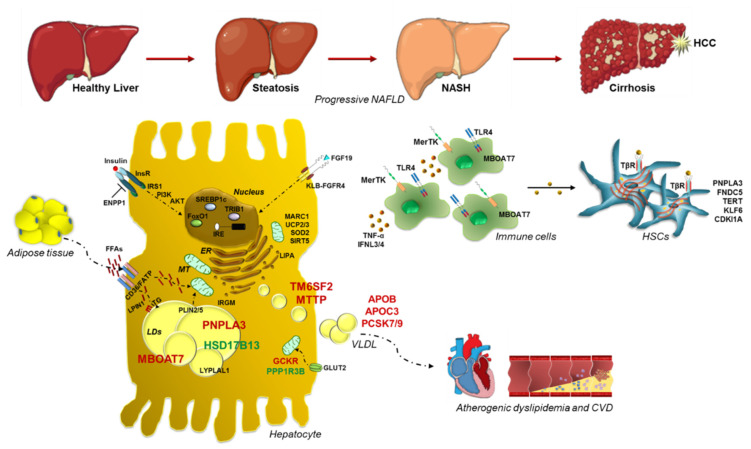
Figure 1.
Impact of genetics in NAFLD pathogenesis and progression towards advanced liver damage. Schematic illustration of the most relevant inherited variations involved in progressive NAFLD, shedding light into their functional effects. PNPLA3, localized at the LD surface in hepatocytes, catalyzes TG hydrolysis. The p.148M variant enhances hepatic TG content upon mutant protein accumulation, hampering TG turnover and dismissal. TM6SF2 is implicated in VLDL formation in ER and release, whereas MBOAT7 transfers arachidonoyl-CoA to Lyso-PI, maintaining membrane fluidity. Their variations dampen VLDL secretion and membrane dynamism, respectively. Viceversa, genetic variants in HSD17B13 and PPP1R3B may exert a protective effect against NAFLD. Heritable variations may also influence glucose and insulin signaling, FFA uptake, fat deposition and VLDL turnover, precipitating fatty liver. In addition, IR and elevated FFAs derived from adipose tissue lipolysis exacerbate fat depot formation induced by genetic modifiers, even activating DNL. Recently, common SNPs in modulators of mitochondrial (MT) function have been proposed as active players in the switching from steatosis to NASH and fibrosis, further corroborating the role of organelle abnormalities in these processes. Furthermore, variants in genes regulating inflammatory response and HSCs activation may precipitate fatty liver to worsened conditions. Finally, genetically determined perturbations in circulating lipids may trigger cardiovascular comorbidities. Dotted lines refer to influx and efflux processes into the hepatocyte, whereas solid lines refer to cell activation or to the transition from simple steatosis up to cirrhosis-HCC.