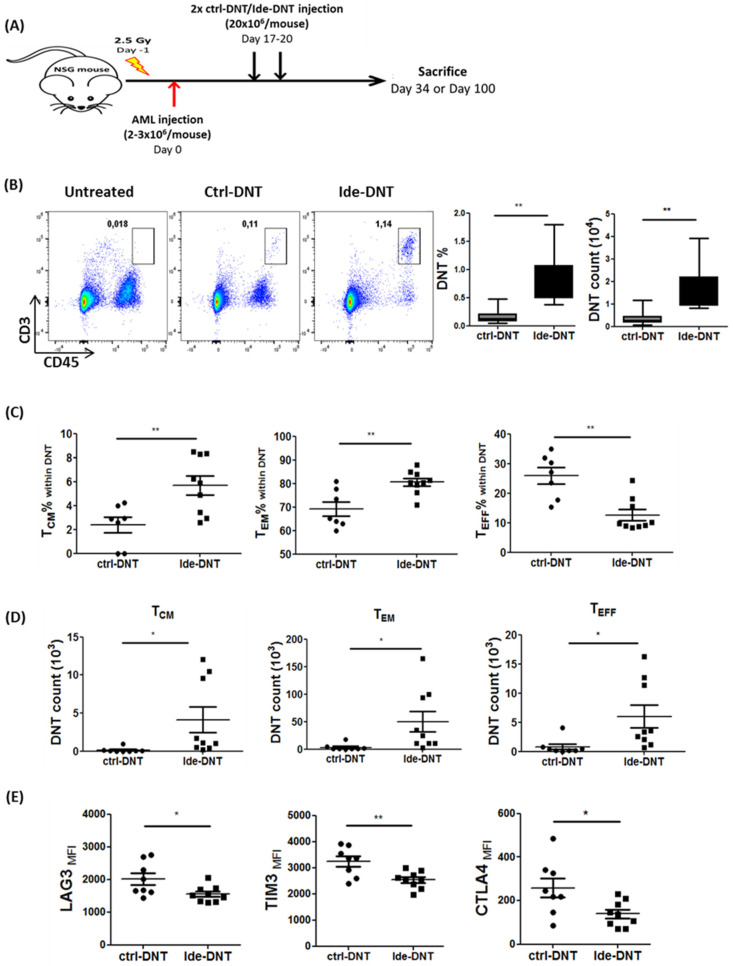
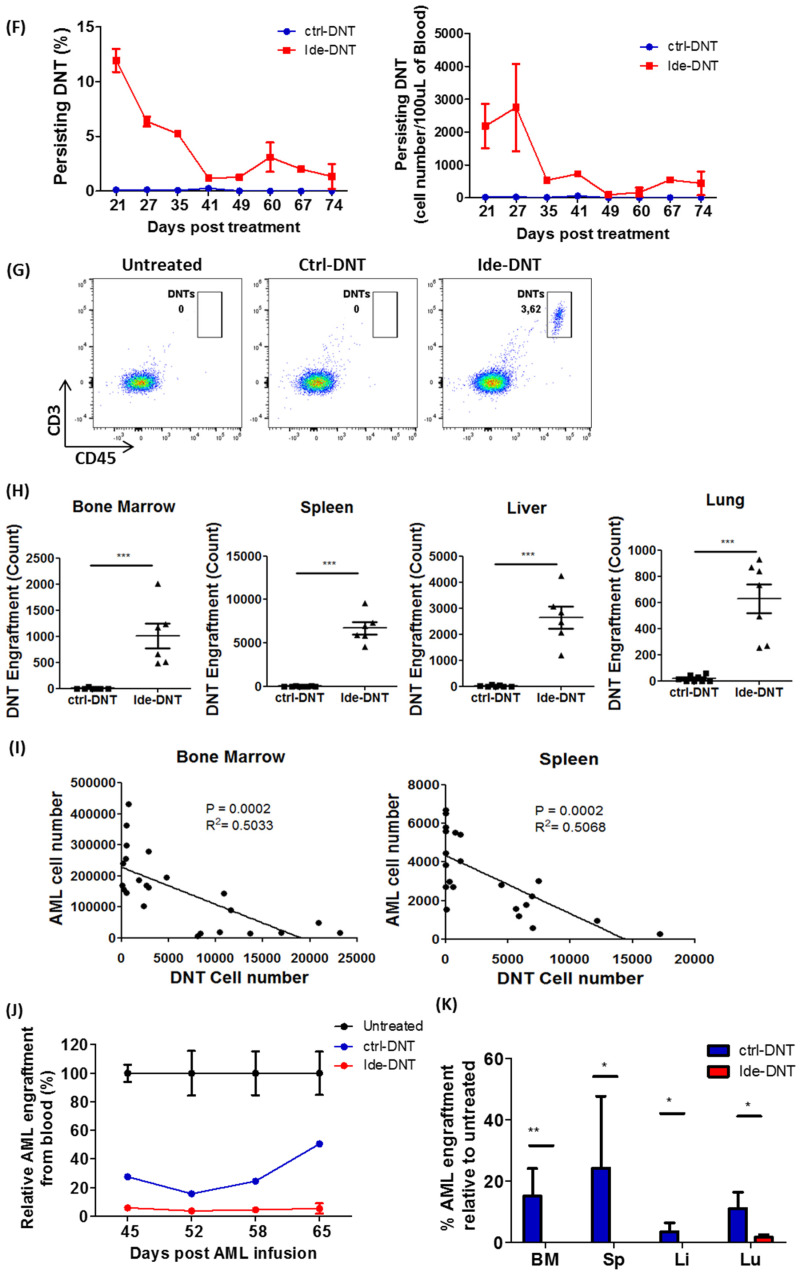
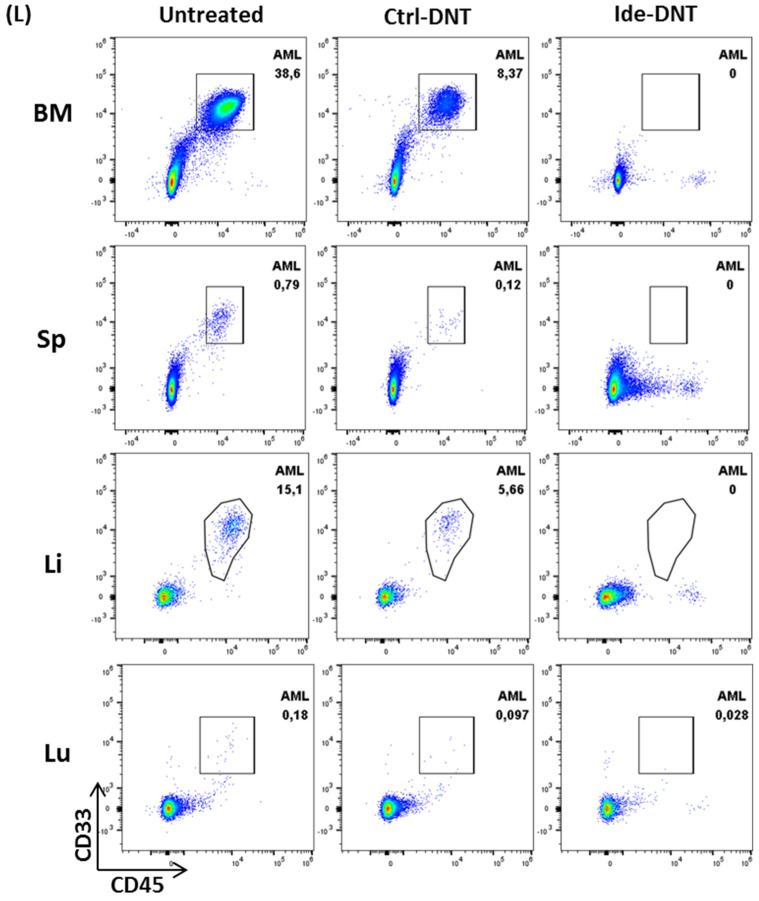
Figure 5.
Ide prolongs persistence of DNT and promotes a durable anti-leukemic effect in vivo. (A) Schematic diagram of patient-derived xenograft (PDX) model used. Sublethally irradiated NSG mice were infused with 2–3 × 106 primary AML patient sample on day 0. On day 17 and 20, mice were infused with 2 × 107 ctrl-DNTs or Ide-DNTs. Mice were sacrificed, and DNT persistence and AML engraftment were assessed in various tissues on day 34 or day 100. (B–E) NSG mice engrafted with the primary AML sample (090543) were treated with PBS (n = 8), ctrl-DNT (n = 8), or Ide-DNT (n = 9), on day 17 and 20 post AML infusion. Subsequently, (B) DNT engraftment in BM (CD45high CD3+), (C) the frequency, (D) and the number of TCM (CD45high CD3+ CD45RA− CD62L+), TEM (CD45high CD3+ CD45RA− CD62L−), and TEFF (CD45high CD3+ CD45RA+ CD62L−), as well as (E) the expression level of the exhaustion markers, LAG3, TIM3, and CTLA4, within the DNT population were determined on day 34, which was 14 days post-treatment using flow cytometry. Each dot represents one mouse. The result is representative of 2 similar experiments with different AML primary blasts. (F–H) Sublethally irradiated NSG mice were engrafted with primary AML sample (090517), followed by PBS, ctrl-DNT, or Ide-DNT treatment on day 17 and 20. (F) Subsequently, the frequency (left) and number (right) of DNTs in peripheral blood were determined between day 21–74 post last DNT infusion in ctrl-DNT and Ide-DNT using flow cytometry. (G) Representative flow plots show DNT persistence from peripheral blood on day 94, which was 74 days post-treatment. (H) Number of viable DNT engrafted in the bone marrow, spleen, blood, liver, and lung on day 100, which was 80 days post-treatment. Each dot represents one mouse. (I) Correlative analysis of DNT and AML cell numbers in the bone marrow (left) and spleen (right) in AML-engrafted mice treated with ex vivo expanded DNTs. The results are representative of three independent xenograft experiments done using AML cell line, MV4-11 (n = 1), and two primary AML patient samples. (J) NSG mice engrafted with the primary AML sample (090543) were treated with PBS, ctrl-DNT, and Ide-DNT on day 17 and 20. Subsequently, the AML level in mice peripheral blood relative to the untreated group was determined on day 45, 52, 58, and 65. (K,L) NSG mice engrafted with the primary AML sample (090517) were treated with PBS (n = 5), ctrl-DNT (n = 7), and Ide-DNT (n = 6) on day 17 and 20. (K) Subsequently, % AML engraftment relative to untreated were determined on day 100, which was 80 days post-treatment. (L) Representative flow plots show AML engraftment (CD45low+ CD33+) from the BM, spleen, liver, and lung. * p < 0.05, ** p < 0.01, *** p < 0.001.