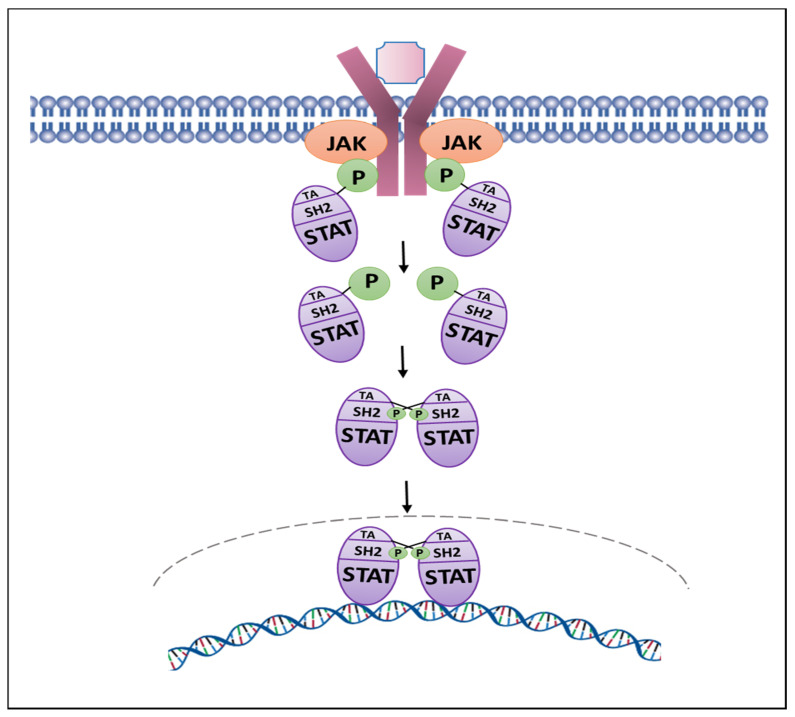
Figure 1.
The JAK-STAT pathway. STAT activation is initiated by the binding of an extracellular ligand to trans-membrane receptors, which bring receptor-associated JAKs into close proximity, leading to their activation. The activated JAKs phosphorylate tyrosine residues that serve as docking sites for cytoplasmic STAT transcription factors. STATs bind to JAK through their Src homology 2 (SH2) domain and JAK tyrosine kinases phosphorylate conserved tyrosine residue located between the SH2 domain and the C-terminal transactivation domain (TA) that result in the formation of STAT dimers, which are stabilized by reciprocal phosphotyrosine and SH2 domain interactions. Dimerized STAT proteins translocate to the nucleus to regulate the transcription of their target genes.