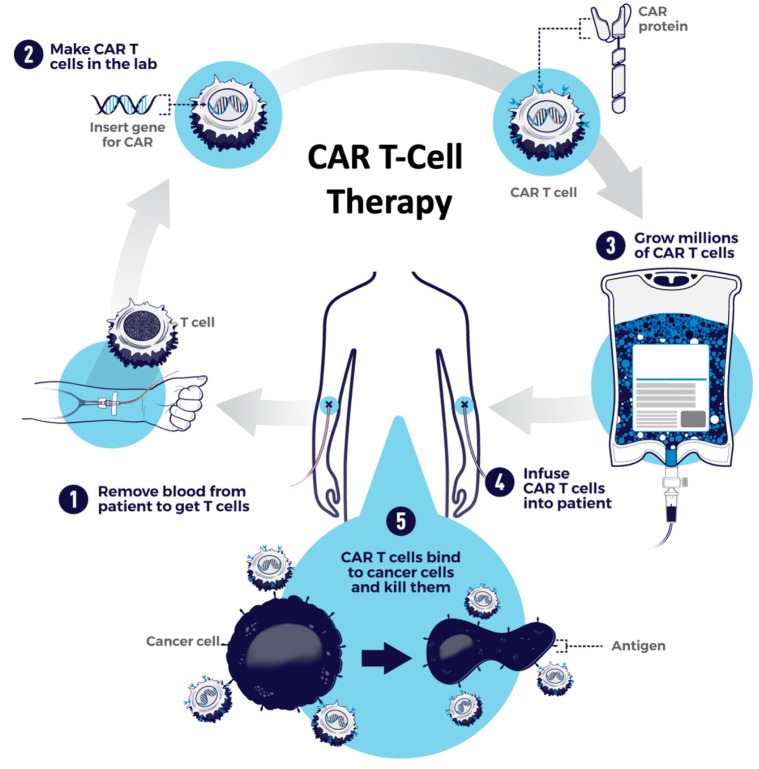
Figure 1.
The general manufacturing process of CAR-T cells, involving (1) leukapheresis, (2) transportation of T-cells to a GMP facility where they are genetically modified and transduced with the CAR, (3) are expanded, purified and undergo quality checks, (4) then are transported back to the treating facility where the patient is treated with lymphodepleting chemotherapy and then infused with the CAR-Ts, (5) which then engage with cancer cells by recognition of the target antigen to mediate the therapeutic effect. Credit: NIH-NCI, open source image.