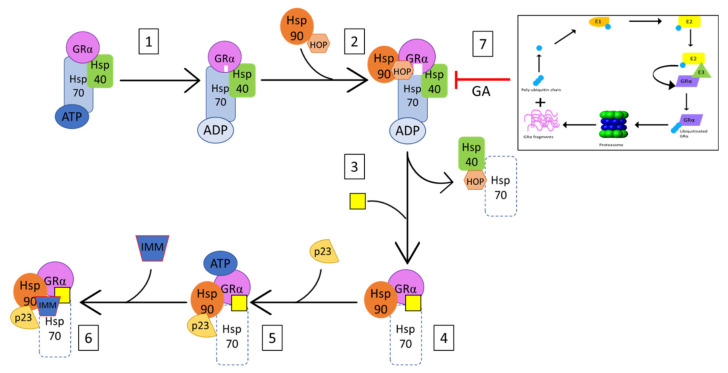
Figure 5.
Simplified mechanism of GRα-Hsp90 association as adapted from Pratt et al. (145). (1) Hsp70 binds GRα in a Hsp40- and ATP-dependent manner, in order to facilitate the priming of the ligand-binding cleft (indicated in white) and the subsequent association of Hsp90 and Hop (2) Association of Hsp90 and Hop to the GRα-Hsp70 complex, results in the complete opening of the receptor’s ligand binding cleft (indicated in white), which allows for (3) the dissociation of Hsp40, Hop, as well as some of the Hsp70, and (4) the subsequent ligand-binding of the receptor (indicated by yellow rectangle). (5) Bound to Hsp90, GRα is now in its ATP-dependent conformation, allowing for the recruitment of p23, which stabilizes and prevents dissociation of the GRα-Hsp90 complex. (6) The dissociation of Hop at step 3 allows for the association of immunophilins (IMM) to the vacant tetratricopeptide repeat (TRP) acceptor site of bound Hsp90. Additionally, (7) Inhibition of GRα-Hsp90 heterocomplex formation, by GA results in the UPS-mediated degradation of GRα.