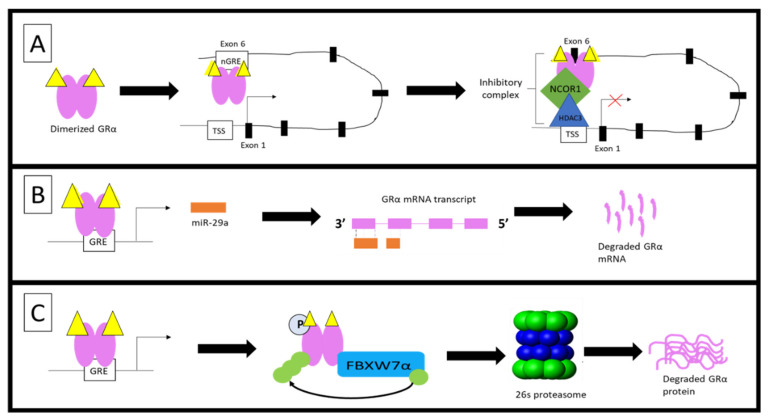
Figure 6.
Dimerization-dependent regulation of GRα levels. Recent studies have revealed three mechanisms through which GRα dimerization increases receptor downregulation. (A) Ligand-induced dimerization enables the binding of GRα to a nGRE site located in exon 6 of the GR gene (NR3C1). Binding of GRα at this site, results in the recruitment of NCOR1 and HDAC3, which, due to chromatin looping, forms a repression complex at the transcription start site (TSS) of the gene, effectively, repressing GRα mRNA transcription [68]. (B) Dimeric GRα binds to a GRE site within the miR-29a promoter, resulting in the transcription of miR-29a. In turn, miR-29a associates with the 3′UTR region of the mature GRα mRNA transcript, resulting in the destabilization and degradation of GRα mRNA [176]. (C) Following ligand binding, hyperphosphorylated GRα interacts with the E3 ligase, FBXW7α, which tags the receptor for degradation by attaching ubiquitin molecules to specific lysine residues. GRα protein degradation occurs via the 26s proteasome [69,121].