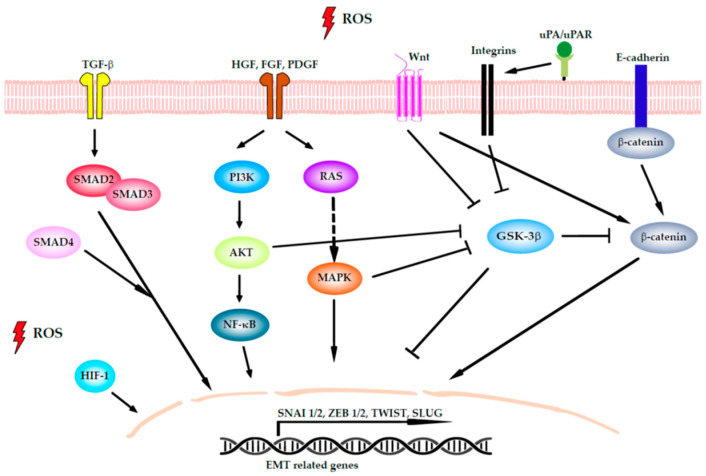
Figure 2.
Signaling networks that regulate epithelial-to-mesenchymal transition (EMT) in colorectal cancer (CRC). The signaling pathways can induce EMT by the activation of the transcription factors (TFs) SNAI1/2, ZEB1/2, TWIST, and SLUG. Transforming growth factor β (TGF-β) induces EMT by phosphorylation of Smad2 and Smad3, which localize to the nucleus with Smad4 to activate EMT TF. Wnt inhibits glycogen synthase kinase-3 (GSK3) to stabilize β-catenin. When β-catenin is active, it translocates to the nucleus to directly activate ZEB1 and SNAI1. Several growth factors that act through tyrosine kinase receptors, such as platelet-derived growth factor growth factor (PDGF), fibroblast growth factor (FGF), and hepatocyte growth factor (HGF), promote EMT through the RAS/mitogen-activated protein kinase (MAPK) signaling cascade, the phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt) axis, and nuclear factor-κB (NF-κB). Both pathways inhibit GSK3 as well as integrin activation, thus cooperating with Wnt signaling. The urokinase plasminogen activator (uPA) bonds to its specific cellular receptor (uPAR) to concentrate proteolytic activity at the cell surface, with this being important for extracellular matrix remodeling. Finally, the E-cadherin present on cell surface binds to cadherins on adjacent cells, whereas its intracellular region contains binding sites to interact with catenins and other regulatory proteins. When the E-cadherin/β-catenin complex is disrupted, it not only affects epithelial integrity but also the Wnt-signaling pathway. Hypoxia-inducible factor 1 (HIF-1) is activated by intracellular ROS and modulates EMT TF activity.