Figure 4.
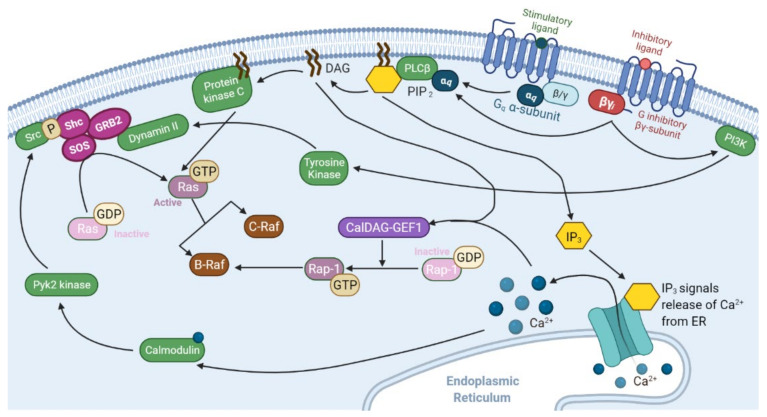
G protein-coupled receptor subunits Gαq and Gβγi interaction with the Ras-Raf pathway. G-proteins are heterotrimeric guanine nucleotide binding proteins with α-, β-, and γ-subunits. When a ligand binds to the extracellular portion of the receptor, it confers a guanine nucleotide exchange factor confirmation that induces the α-subunit to exchange its bound GDP to GTP. This causes the α-subunit to disassociate from the receptor and βγ- subunit. Both the α and βγ subunits effect changes in the cell, before the α subunit hydrolyzes the GTP and returns the receptor complex to its inactive state [94,95,107,108]. Both αq and βγi activate phospholipase C-β (PLCβ) to create the second messengers of diacylglycerol (DAG) and inositol-1,4,5-triphosphate (IP3) through the hydrolyzation of phosphatidylinositol 4,5-bisphosphate (PIP2) [109]. DAG activates PKC, which directly phosphorylates and activates Ras proteins [110,111]. IP3 stimulates the calmodulin pathway and Pyk2 kinase by way of inducing calcium release from the endoplasmic reticulum [112]. The resulting phosphorylation provides the base for Shc anchoring and recruitment of Ras’ guanine exchange factor complex [113]. Both DAG and IP3 play a role in allosterically controlling CalDAG-GEF1, a guanine exchange factor for Rap1 [94,114]. Once Rap1 has exchanged its bound GDP for GTP, it can activate BRaf in the place of Ras [115]. Furthermore, βγi activates phosphoinositide 3-kinase (PI3K), which augments cell signaling from tyrosine kinase receptors to increase Dynamin II [116], an additional anchor for Shc and Ras’ GEF complex [117,118,119].