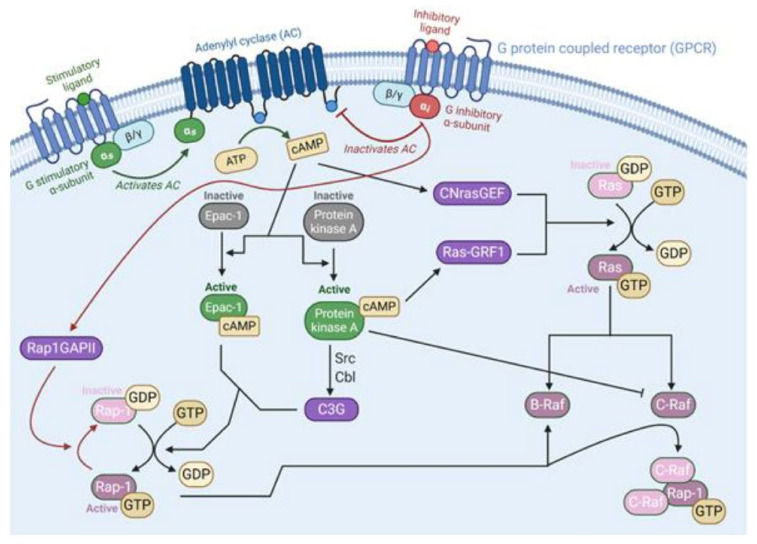
Figure 5.
G-protein-coupled receptor subunits Gαs and Gαi interaction with the Ras-Raf pathway. When activated by a ligand, the GPCR is induced to exchange its bound GDP for GTP, freeing the α subunit to act on the cell. αs acts on adenylyl cyclase (AC) to increase the production of the second messenger cAMP from ATP [120]. In turn, cAMP activates Epac-1 [121] and protein kinase A [120]. Epac-1 and C3G, a downstream molecule from PKA, are guanine-nucleotide exchange factors for Rap-1 and induce the exchange of GDP to GTP to activate it [121,122]. Both activate Rap-1, which can modulate the activity of BRaf [115]. Protein Kinase A has other mechanisms of action as well. It can directly prevent the activation of CRaf through phosphorylation. Both activate Rap-1, which can modulate the activity of BRaf [115]. Protein kinase A has other mechanisms of action as well. It can directly prevent the activation of CRaf through phosphorylation [123]. It also activates a Ras GEF, RasGRF1, to start the MAPK cascade in certain cells [124]. In contrast, αi inhibits adenylyl cyclase and produces opposite effects. It also activates a Ras GEF, RasGRF1, to start the MAPK cascade in certain cells [124]. In contrast, αi inhibits adenylyl cyclase and produces opposite effects.