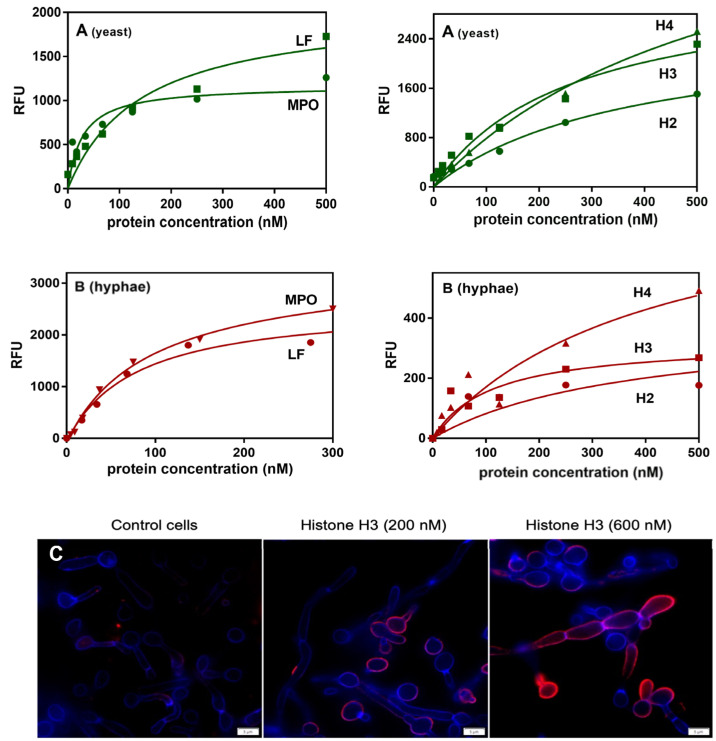
Figure 3.
Interactions of selected NET proteins with the yeast and hyphal forms of C. albicans cells. The interactions of commercially available NET-composing proteins were tested using flow cytometry analysis (A) for the yeast-like form, and the microplate ligand-binding assay (B,C) for the hyphae. In the flow cytometry experiments, 1.3 × 106 C. albicans cells were incubated in the presence of selected proteins, previously labeled with NHS-fluorescein. The results were presented as a mean fluorescence intensity per cell. In the microplate assay, the binding of fluorescein-labeled NET proteins was tested towards C. albicans hyphal forms (1 × 106 cells) grown in RPMI 1640 medium for 3 h at 37 °C. After washing and blocking the unbound surfaces, the cells were incubated with labeled NET proteins. The amount of bound proteins was determined by measurements of the fluorescence intensity (excitation and emission wavelengths of 488 nm and 525 nm, respectively) with a multi-mode microplate reader. For all of the experimental data, the protein binding analysis was performed using GraphPad Prism software and a saturation one-site binding model. The representative result of three experiments performed in duplicates is presented. The microscopic analysis (Olympus IX73 microscope) of histone H3 binding to the surface of C. albicans cells was performed, after the cell fixation, using mouse primary anti-H3 antibodies and Alexa Fluor 555-conjugated secondary antibodies. The fungal cell wall was labeled with Calcofluor White dye.