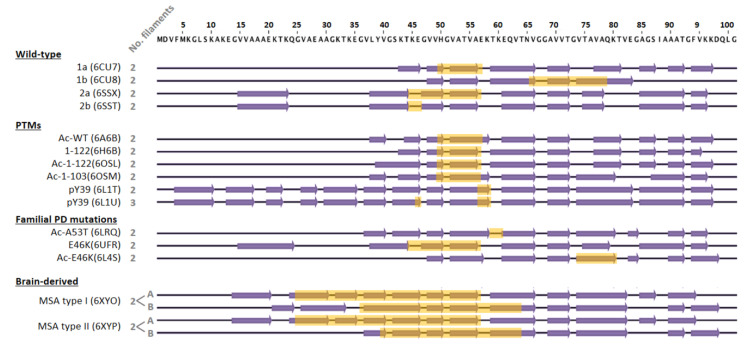
Figure 3.
Schematic representation of amino acids 1–100 of α-syn, comparing the secondary structure elements that form the protofilament cores within the different fibril structures solved by cryo-EM. The structures of wild-type, post-translationally modified species, and familial PD mutants were obtained from recombinantly expressed α-syn. In these cases, fibrils were composed of two identical protofilaments or three in the case of fibrils phosphorylated at tyrosine 39 (pY39). The interface between protofilaments is highlighted in yellow. For the WT protein sequence, four polymorphs have been found with three different β-sheet arrangements. Polymorphs 2a and 2b have the same β-sheet arrangement, but differ in the protofilament interface. Regarding PTMs, both N-terminal acetylation and C-terminal truncation seem to have little effect on the secondary structure of the fibril, with a comparable β-sheet arrangement to polymorph 1a. Similarly, neither H50Q nor A53T mutations significantly disturb the 1a fold, only changing the pairing geometry of the protofilaments. In the case of the E46K mutation, two structures have been published, one similar to the 2a polymorph and another one, acetylated at the N-terminus, with a different and more stable fold. Structures of two distinct fibrils (type I and II) derived from brains of deceased MSA patients differ from those obtained in vitro. In these cases, fibrils are composed of two different protofilaments (A and B) that interact with each other through different residues and show much more extended interfaces. β-strand elements were considered as in Schweighauser et al. (2020).