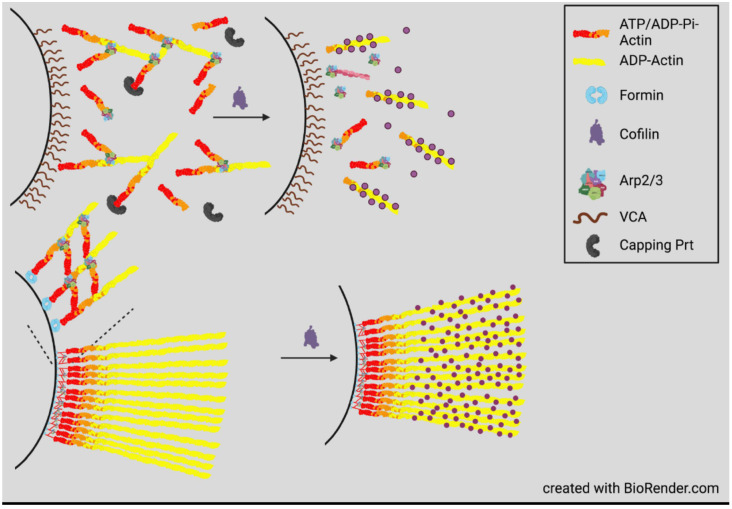
Figure 2.
Effects of cofilin on different actin filament systems in vitro. (Top) Diagrammatic representation of an Arp2/3 nucleated branched actin filament network at the surface of a bead with an attached Arp2/3 activator showing activation of Arp2/3 complex and nucleation of filaments, which bind through the complex to the side of older filaments to generate a branched network. Filaments are disassembled by cofilin as they age (loss of Pi) relative to ADP-actin and show array treadmilling. Capping protein limits growth to short filaments; profilin (not shown) sequesters monomers to prevent spontaneous nucleation; CAP1 (not shown) enhances turnover of cofilin-actin fragments. Under low ionic strength conditions, the branched network is rapidly disassembled even by low cofilin concentrations and will not assemble above 50 nM cofilin [51]. (Bottom) Cofilin can maintain a steady state dynamic actin network mediated from both Arp2/3 complex and formin-nucleated filaments in the absence of cofilin post-translational regulation. Beads maintain a narrow band of Arp2/3 complex branched filaments along their surface with long, linear formin-nucleated filaments extending in a halo and binding excess cofilin. Beads start rotating, probably because of asymmetry in assembly, and maintain rotation. Based on data from Bleicher et al. [51].