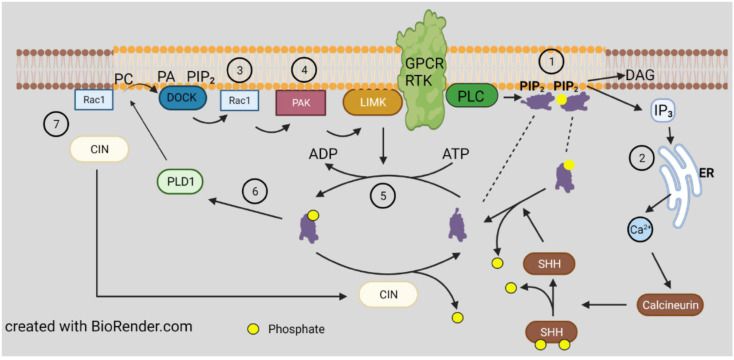
Figure 3.
Membrane organization of cofilin regulatory proteins and lipid binding sites. Membranes associated phosphatidylinositol phosphates (PIPs), found in highest concentrations in sphingolipid/cholesterol enriched lipid raft domains, bind both cofilin and serine 3 phospho-cofilin ①, which are released upon activation of PLC. Different isoforms of phospholipase C (PLC) can be activated by receptor tyrosine kinases (RTKs, diagrammed here) or by G-protein coupled receptors (GPCRs) and release diacylglycerol (DAG) and various phosphoinositols. ② Inositol 1,4,5 triphosphate (IP3), released from PI(4,5)P2, (PIP2) is an activator for release of intracellular Ca2+. Both GPCRs and RTKs also activate pathways for stimulating guanine nucleotide exchange factors (GEFS) for activating Rho family GTPases (e.g., Rac1) ③, which work at the plasma membrane to activate PAKs ④ by binding to their autoinhibitory domain to permit autophosphorylation that activates the PAK. Active PAK phosphorylates and activates LIMK1, also membrane bound, to locally inactivate cofilin ⑤. Cofilin phosphorylated on S3 is an activator of phospholipase D1 ⑥, which converts phosphatidylcholine, a major membrane phospholipid, to choline and phosphatidic acid (PA) [61]. PA can further signal through binding to other membrane proteins, such as those with DOCK domains, to enhance or reduce downstream signaling. The cofilin phosphatase chronophin (CIN) is recruited to the leading edge of cells through a Rac1 ⑦ and PI3-kinase dependent pathway [62].